The following is the original English version of Chapter 3 that was translated into Spanish by Pilar Catalán with assistance from Miguel A. García.The Spanish citation is:Nickrent, D. L. 2002. Orígenes filogenéticos de las plantas parásitas. Capitulo 3, pp. 29-56 en J. A. López-Sáez, P. Catalán and L. Sáez [eds.], Plantas Parásitas de la Península Ibérica e Islas Baleares. Mundi-Prensa, Madrid.The English citation is:Nickrent, D. L. 2002. Phylogenetic Origins of Parasitic Plants. Chapter 3, pp. 29-56 In J. A. López-Sáez, P. Catalán and L. Sáez [eds.], Parasitic Plants of the Iberian Peninsula and Balearic Islands. Mundi-Prensa, Madrid. |
Throughout its history, the field of plant systematics has undergone changes in response to the advent of new philosophical ideas, types of data, and methods of analysis. It is no exaggeration to say that the past decade has witnessed a virtual revolution in phylogenetic investigation, owing mainly to the application of molecular methodologies and advancements in data analysis techniques. These powerful approaches have provided a source of data, independent of morphology, that can be used to address long-standing questions in angiosperm evolution. These new methods have been applied to systematic and phylogenetic questions among parasitic plants (Nickrent et al. 1998), but have often raised as many new questions as they have solved, in part due to the amazingly complex nature of the genetic systems present in these organisms. The goal of this chapter is to provide a general synopsis of the current state of understanding of parasitic plant phylogeny. To place in context results concerning the parasites, it is necessary to first examine general features of angiosperm phylogeny.
Angiosperm Phylogeny - Background from a Molecular Perspective
Although the focus of this chapter will be mainly on results
from molecular phylogenetic analyses, it must be mentioned that
none of these studies would have been possible in the absence
of evolutionary hypotheses first proposed from morphological data.
Many of the relationships among angiosperms as viewed by Cronquist
(1988), Takhtajan (1997) and Thorne (1992) have been supported
using molecular characters. For a 1999 update to the Thorne system,
see web posting by (Reveal 1998). Elucidating phylogenetic
relationships
among the approximately 460 angiosperm families has been advanced
by molecular analyses incorporating hundreds of taxa, the first
of which utilized the chloroplast gene rbcL (Chase et al.
1993). In cases where the molecular trees differed markedly from
"traditional" morphology-based trees, a justification
frequently given for accepting the traditional concept was that
the gene tree is simply the result of analyzing a single "character"
whereas the morphological tree was derived from many. Whether
the gene or the nucleotide is the character can be debated; however,
this criticism stimulated researchers to begin sequencing additional
genes, e.g. chloroplast atpB (Hoot et al. 1995) and nuclear
small subunit ribosomal DNA (SSU rDNA) (Nickrent and Soltis 1995,
Soltis et al. 1997). It had also become apparent at that greater
resolution could be obtained by simultaneously analyzing combinations
of genes derived from different subcellular compartments. A study
using SSU rDNA, chloroplast atpB, and rbcL (Soltis
et al. 1998, Soltis et al. 1999) resulted in a generally well-supported
topology for the major clades of angiosperms. The topology of
the basal portion of the angiosperm tree was further resolved
by two independent phylogenetic studies that analyzed data sets
combining five genes from the nucleus, chloroplast and mitochondrion
(Parkinson et al. 1999, Qiu et al. 1999). An exciting finding
was that all of these studies unambiguously identified the monospecific
New Caledonian tree Amborella as sister to the remaining
angiosperms. Instead of working in isolation, all of these multigene
studies were collaborative efforts involving many people from
distant laboratories. The culmination of such an effort can be
seen in an ordinal classification of angiosperms by the Angiosperm
Phylogeny Group (APG 1998).
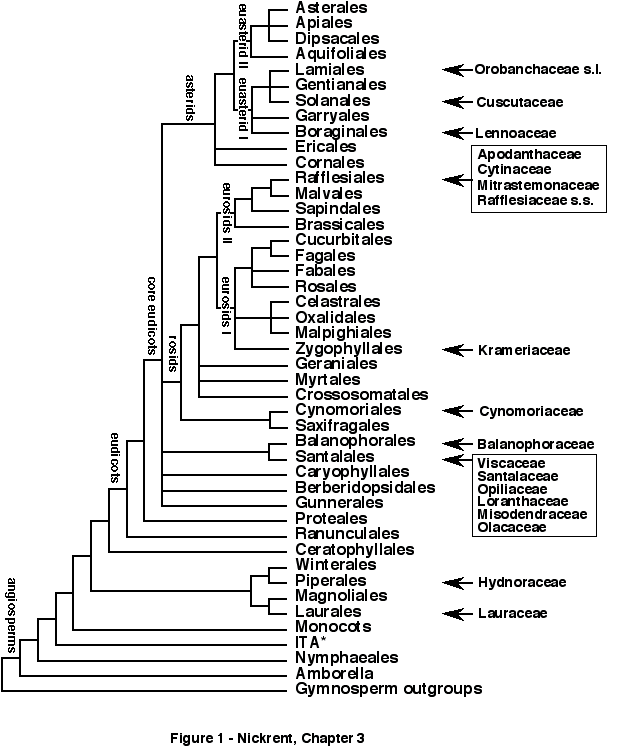
It now appears that the general topological features of the angiosperm phylogenetic tree have been identified. For example, the primary division among angiosperms is not Dicotyledonae and Monocotylendonae, for indeed the latter are derived from within a group of dicots that predominately have monosulcate pollen, (i.e. Magnoliidae in part according to Cronquist (1981)). Emerging from this grade of monosulcate families is a well-supported clade termed the eudicots that have triaperturate or triaperturate-derived pollen (Chase et al. 1993, Donoghue and Doyle 1989). The phylogeny of eudicots has been greatly clarified from combined analyses of multiple genes (Hoot et al. 1999) that show Ranunculales and Proteales to be basal eudicots, not magnoliids. Strong support is also obtained for the "core eudicots," a group composed of Caryophyllales, Santalales, Saxifragales, rosids and asterids (Figure 1).
Current Concepts of Parasitic Plant Phylogenetic Relationships
Given the above advancements in our understanding of angiosperm
relationships, it is now timely to examine the classification
of parasitic angiosperms from a new phylogenetic perspective (Figure 1). As stated in Nickrent et al.
(1998), the placement of some parasitic plant families within
the global angiosperm phylogeny is not disputed, such as Orobanchaceae
s. lat. (including part of Scrophulariaceae, see below),
Convolvulaceae,
Lennoaceae, and Lauraceae (Cassytha). In contrast, there
are several hemiparasitic (e.g. Krameriaceae and Santalales) and
holoparasitic (e.g. Balanophorales and Rafflesiales) taxa that
have not been satisfactorily placed in the angiosperm phylogeny.
The latter two orders have been dubbed the "nonasterid holoparasites"
because they are clearly not related to asterid holoparasites
such as are found in Orobanchaceae, Lennoaceae, and Convolvulaceae
(Nickrent et al. 1998) and are also not closely related to each
other. As detailed below, classification of phylogenetic reconstruction
for the nonasterid holoparasites has proven difficult and controversial.
In the following section, parasitic plant families are classified in nine orders and the discussion for each incorporates data from morphological and molecular work (when available). The authorities for the higher taxa are from Reveal (1998). Whereas the first seven orders are very likely "natural" in that they represent independent evolutionary events, monophyly of the last two orders is not certain being derived from preliminary molecular analyses. For each taxonomic group, the phylogenetic position of the order within angiosperms and phylogenetic relationships within the order will be discussed.
A. Laurales Perleb (1826). Lauraceae Juss., nom. cons. (1789). Cassytha is the sole genus in Lauraceae that has evolved the parasitic habit. Moreover, because of its resemblance to Cuscuta (Convolvulaceae), Cassytha represents one of the most remarkable cases of parallelism in the angiosperms. Despite the radical departure of its vegetative state from the typical condition in Lauraceae (trees and shrubs), floral morphology in Cassytha makes assignment to this family unequivocal. As pointed out by Kuijt (1969) this discrepency is a good example of mosaic evolution. Cassytha has been segregated into its own family (Cassythaceae Bartl. ex Lindl., nom. cons. (1833)), however, all modern classifications place the genus in Lauraceae (Heo et al. 1998, van der Werff and Richter 1996). The most recent systematic revision of the genus (Weber 1981) suggests Cassytha contains about 20 species, 15 of these are Australian with a few in Africa and Asia and one pantropical species (C. filiformis). Most research on Cassytha has centered around the morphology of the haustorium (Cartellieri 1928, Heide-Jørgensen 1987), seed and fruit anatomy (Rao 1980), embryology (Sastri 1956, Sastri 1962), and host range (Nayar and Nayar 1952, Werth et al. 1979). At this writing, no rbcL or nuclear SSU rDNA sequences for Cassytha have been deposited with Genbank. A molecular phylogeny generated from the chloroplast gene matK showed Cassytha to be relatively divergent but still a component of the family (Rohwer 2000). Sequences from three chloroplast genes and nuclear large-subunit rDNA were obtained for 44 of the 50 genera of Lauraceae and phylogenetic analyses placed Cassytha in Lauraceae (Renner and Chanderbali 2000). The same result was obtained using the mitochondrial genes cox1 and atpA (Barkman et al. 2000).
B. Hydnorales Takht. ex Reveal (1992). Hydnoraceae C. Agardh, nom. cons. (1821). Hydnoraceae contain two genera, Prosopanche (of South America) and Hydnora (of Africa and Madagascar). Musselman and Visser (1986) elected Hydnora as being the "strangest plant in the world," and this epithet is certainly deserving given the highly modified vegetative and floral morphology exhibited by these holoparasites. Hydnoraceae are the only flowering plants known that lack leaves (or modifications such as scales). Two types of roots exist in Hydnora: horizontal rhizome-like "pilot roots" that are hexagonal in cross section and vermiform outgrowths from the ridges of the pilot roots called haustorial roots whose function is to attach to the host. The epigynous flowers are composed of three or four fleshy, valvate tepals that fuse with the staminal filaments to form a tepalostemon (perianth tube).
The position of Hydnoraceae within the global angiosperm phylogeny has been the source of much disagreement in the literature. Cronquist (1988) placed Hydnoraceae in Rafflesiales, an order thought to be related to Santalales of subclass Rosidae. The placement was influenced by the parasitic habit and supposed shared derived features of the flower. The system of Takhtajan (1997) placed the family in its own order, allied to Rafflesiales. In contrast to Cronquist but in agreement with Thorne (1992), both orders were classified in Rafflesianae within the mainly monosulcate subclass Magnoliidae. It is of interest to note that Hydnora has monosulcate pollen whereas Prosopanche has bisulcate pollen (see p. 700 in Cronquist (1981)). Floral morphology and the presence of unitegmic ovules prompted Coccuci to propose affinities between Hydnoraceae (Prosopanche) and Mitrastema (Mitrastemonaceae - Rafflesiales) (Cocucci 1975, Cocucci 1976). A scheme was proposed whereby Hydnora and then Prosopanche were derived from Mitrastemon which was itself derived from Annonaceae. Takhtajan (1997) stated that superorder Rafflesianae (including Hydnorales and Rafflesiales) originated from ancestors shared with Aristolochiaceae (Asaroideae).
To address the question of phylogenetic position of Hydnoraceae within angiosperms, nuclear SSU rDNA sequences were obtained for Hydnora and Prosopanche and analyzed with over 200 other angiosperm sequences (Nickrent and Duff 1996). From this study, Hydnoraceae emerged as monophyletic and sister to the "paleoherb" family Aristolochiaceae. Although the chloroplast gene rbcL is not present in Hydnoraceae (Nickrent et al. 1997b), mitochondrial genes such as atpA and matR are present. Analyses of these genes separately and in a combined data set that included nuclear SSU rDNA and plastid rbcL and atpB (the latter two genes coded as missing for Hydnoraceae) support the paleoherb status of the family (A. Blaerer, Y.-L. Qiu, D. Soltis, D. Nickrent, unpublished data). These data also support the evolution of Hydnoraceae independent from Rafflesiaceae and Mitrastemonaceae. Thus, it is appropriate to acknowledge and commend the insights made by the 19th century botanist Solms-Laubach (1894) whose original general phylogenetic placement near Aristolochiaceae is now substantiated with genetic data.
C. Santalales Dumort. (1829). Taxonomic classifications of the sandalwood order, Santalales, vary with respect to circumscriptions of the component families as well as its relationship to other angiosperms. Two monospecific and nonparasitic families, the Medusandraceae (Medusandra richardsiana) and the Dipentodontaceae (Dipentodon sinicus), have been variously placed in or near Santalales. Cronquist (1981) included Dipentodontaceae in Santalales whereas it was classified with Violales by others (Takhtajan 1997, Thorne 1992). Although all modern classifications indicate a relationship between Medusandraceae and Santalales, the family is likely best placed with Flacourtiaceae or Euphorbiaceae(Malpighiales). Kuijt (1968) did not comment on the affinities of these families; however, it can be assumed that their omission indicates he does not consider them a part of Santalales. For this chapter, Santalales will be assumed to contain six families: Olacaceae, Misodendraceae, Loranthaceae, Opiliaceae, Santalaceae, and Viscaceae. The mistletoe family Eremolepidaceae (Kuijt 1988) is treated as a component of Santalaceae based upon molecular analyses (Nickrent and Duff 1996, Nickrent et al. 1998). It is likely that continued research will require additional changes in classification of this order (e.g. Olacaceae, see below).
The position of Santalales within the global angiosperm phylogeny remains unresolved, despite the three-gene study reported by the APG (1998) and Soltis et al. (2000). The order emerges from a large polytomy at the base of the "core eudicots" along with Caryophyllales, Gunnerales, rosids and asterids. This position suggests that Santalales are not related to more derived rosids, as portrayed in most traditional classifications, but that they are an older lineage. This concept is in line with that of Sleumer (1984a) who viewed Olacaceae as an ancient family that differentiated prior to the separation of the continents during the Cretaceous. It is also worth considering that primitive Santalales (e.g. Olacaceae) may share with Caryophyllaceae a pseudodiplostemonous androecium whereby pentamerous flowers are derived (via reduction) from trimerous prototypes (Ronse Decraene et al. 1998).
1. Olacaceae Mirb. ex DC., nom. cons. (1824). Olacaceae, composed of ca. 28 genera, all with tropical or subtropical distributions, appears to be the only family in the order that contains both autotrophic and root hemiparasitic genera. Unfortunately, information on the parasitic habit is known only for 12 of the 28 genera. It has long been considered the least specialized family in Santalales as reflected by the phylogenetic schemes presented by Fagerlind (1948) and Kuijt (1968). Sleumer (1984b) divided the family into three subfamilies: Anacolosoideae, Olacoideae, and Schoepfioideae. Olacaceae is difficult to define because extreme variation exists in many morphological features. For this reason, it is not surprising that many segregate families have been proposed, most notably Erythropalaceae and Octoknemaceae (Baas 1982).
Past published molecular phylogenetic analyses using nuclear SSU rDNA and plastid rbcL have confirmed the basal position of the family within Santalales (Nickrent and Duff 1996, Nickrent et al. 1998, Nickrent and Franchina 1990), but conclusions about intergeneric relationships were limited by lack of sufficient taxon sampling. More recently (Nickrent and Malecot 2000), sequences for SSU rDNA and rbcL for 18 genera were used in a combined analysis. As seen previously, Schoepfia does not appear to be related to Olacaceae but to Misodendrum (Misodendraceae), albeit on a long branch, that connects to Loranthaceae. These data support the segregation of this genus into its own family (Schoepfiaceae Blume 1850). Some features of the subfamilial classification of Sleumer (1984) are supported by molecular data, such as tribe Couleae, whereas tribes Olaceae and Anacolosae will require different circumscription to conform to phylogenetic results. For example, Malania, thought by some to belong to tribe Olaceae, is closely related to Ximenia (tribe Ximeniae) based upon morphological and molecular characters (Nickrent and Malecot 2000). As traditionally defined, Olacaceae is certainly paraphyletic and, with additional molecular work on problematic genera such as Brachynema, Erythropalum, and Octoknema, may turn out to be polyphyletic.
2. Misodendraceae J. Agardh (1858). This distinctive family is composed of one genus (Misodendrum) of ca. eight species. These mistletoes parasitize southern hemisphere beech trees (Nothofagus) in South America. The genus is characterized by the plumose staminodes on the fruits that function in wind-dispersal. The phylogenetic affinities of the family appear to be with Loranthaceae and, as mentioned above, Schoepfia. This relationship is seen using nuclear SSU rDNA and plastid rbcL separately and in combination (Nickrent and Duff 1996, Nickrent et al. 1998). The molecular data suggest that the common ancestor of Misodendrum, Schoepfia and (primitive) Loranthaceae was the first lineage to evolve from Olacaceae. Because the latter two taxa are root parasites, Misodendrum may represent the first evolutionary "experiment" with the mistletoe habit.
3. Loranthaceae Juss. (1808). Having 75 genera and over 900 species, Loranthaceae is the largest family of Santalales. The family has traditionally been allied with viscaceous mistletoes, however, Barlow (1964) provided compelling evidence from cytology and biogeography that supported the concept of two independent families. In contrast to Linnaeus, who recognized just one genus (Loranthus) for the family, P. van Tieghem erected a plethora of new names, all but 32 of which have been relegated to synonymy. Much of this revisionary work was conducted by B. H. Danser and our modern generic concepts can be traced to his insightful and thorough work (e.g. (Danser 1933)).
The modern era of Loranthaceae systematics includes (but is not limited to) workers such as J. Kuijt (Central and South America), B. A. Barlow (Australia and Malesia), D. Wiens, R. Polhill, and S. Balle (all Africa and Madagascar). The recent publication of Mistletoes of Africa (Polhill and Wiens 1998) is an important contribution given the high generic and species diversity on this continent. At present, no modern subfamilial classification exists that encompasses all genera of Loranthaceae worldwide. Keys and a classification system to the Old World genera were published by Danser (1933) who divided the family into four tribes: Nuytsieae, Elytrantheae, Lorantheae, and Psittacantheae. Barlow (1964) merged the first two tribes, but his scheme was essentially like Danser's. Although a large number of systematic treatments exist, mainly owing to the efforts of J. Kuijt, no higher-order classification has been proposed for the New World genera.
To date, only two molecular phylogenetic analyses of Loranthaceae have been published (Nickrent and Duff 1996, Nickrent et al. 1998). In studies combining nuclear SSU rDNA and rbcL, Loranthaceae emerge as monophyletic. The separate familial status of Loranthaceae and Viscaceae was fully supported by analyses of both genes. Although rbcL sequences have sufficient numbers of substitutions to address phylogenetic relationships within Santalales, it appears that such is not the case for examining intergeneric relationships in Loranthaceae (Nickrent et al. 1998). Analyses utilized nuclear SSU rDNA sequences from 23 loranth genera suggested a major dichotomy between the Old and New World loranths, in general agreement with previous morphology-based classifications. These studies also showed that branch lengths leading to Old World genera were generally longer than those leading to New World genera, likely reflecting the different generic concepts being employed by different workers. Preliminary work using chloroplast matK indicates this gene has greater numbers of substitutions, thus making it a more appropriate molecular marker to address intrafamilial relationships in Loranthaceae (Nickrent, unpublished).
4. Opiliaceae (Benth.) Valeton, nom. cons. (1886). This small family of ten genera and 32 species has traditionally been considered closely related to or a component of Olacaceae, although all modern classifications treat it as distinct. Molecular phylogenetic analyses using nuclear SSU rDNA and rbcL fully support the recognition of a monophyletic family separate from Olacaceae. These analyses, most recently including six of the ten genera, show that the family is most closely related to Santalaceae, particularly if the rbcL sequences are analyzed alone (Nickrent and Duff 1996, Nickrent et al. 1998, Nickrent and Malecot 2000). As discussed in Nickrent et al. (1998), a number of morphological features can be cited that support an association between Opiliaceae and Santalaceae.
5. Santalaceae R. Br., nom. cons. (1810) including Eremolepidaceae Tiegh. ex Nakai (1952). With 40 genera and 490 species, the sandalwood family is second in size only to Loranthaceae for the order. The subfamilial classification of Pilger (1935) has been used as the starting point for more recent revisions and modifications. Most notable are the contributions by Stauffer (Santales-Studien I-X; see listing in Stearn (1972)) who unfortunatley did not publish a revised classification of the family prior to his death in 1965. Currently, the family is divided into four tribes: Anthoboleae (three genera including Exocarpos), Amphorogyneae (11 genera, including stem parasites such as Dendromyza, Dufrenoya, and Phacellaria), Santaleae (= Osyrideae of Pilger; ca. 20 genera), and Thesieae (5 mainly southern hemisphere genera including Thesium). Previous molecular studies of nuclear SSU rDNA and rbcL (Nickrent and Duff 1996, Nickrent et al. 1998) utilized 12 or fewer sequences, hence few statements can be made about intrafamilial relationships. The family is not monophyletic but composed of a paraphyletic assemblage that culminates in Viscaceae (see below). One result of these analyses that appears using the two genes separately or in combination is that the three genera (Antidaphne, Eubrachion and Lepidoceras) placed in the family Eremolepidaceae by Kuijt (1988) are not monophyletic and distinct from Santalaceae but occur within a grouping of taxa traditionally classified as tribe Santaleae. This result is supported by karyological and morphological features (Wiens and Barlow 1971) as well as embryology (Bhandari and Vohra 1983). At present, complete sequences for SSU rDNA and rbcL are available for 17 genera of Santalaceae (Nickrent and Malecot 2000). Although the eremolepidaceous genera presently group with Santaleae, there were no representatives included from tribe Amphorogyneae. It will be of interest to see whether the inclusion of additional stem parasitic Santalaceae (particularly Phacellaria) will change this topology. From the present data, it appears that the viscaceous and eremolepidaceous stem parasites represent multiple independent evolutionary events.
6. Viscaceae Batsch (1802). Viscaceae are a well-defined family composed of six or seven genera and ca. 350 species. In all molecular phylogenetic analyses of the family conducted to date (Nickrent and Duff 1996, Nickrent et al. 1998), Viscaceae are strongly-supported as monophyletic. As stated above, this clade emerges from a paraphyletic assemblage comprising Santalaceae. Viscaceae was subsumed into Santalaceae because the latter is not monophyletic by the APG (1998). Because Viscaceae is monophyletic, well-defined, and economically important, that classification will not be followed and Viscaceae will be recognized as a family for this chapter. This follows the philosophy expressed by the APG which stated "classification is not only a matter of grouping according to the principle of monophyly, but it is also a matter of communication ..." Sequence data for nuclear SSU rDNA and rbcL have been obtained for all genera in the family. In combined analyses, full resolution of generic relationships was not obtained, which is surprising given increased substitution rates in both genes (Nickrent 1996, Nickrent and Soltis 1995). Strongly-supported relationships include Phoradendron with Dendrophthora and Korthalsella with Ginalloa, whereas the Viscum plus Notothixos clade (obtained in the strict consensus tree) does not receive high support (from bootstrap resampling). The position of the dwarf mistletoe (Arceuthobium) relative to the other genera is also unresolved.
Molecular methods have been used to address interspecific relationships for Arceuthobium (Nickrent et al. 1994), Korthasella (Molvray et al. 1999), and Phoradendron (Ashworth 2000a). In the latter study, nuclear large-subunit rDNA as well as the trnL-trnF chloroplast spacer (Ashworth 2000b) were sequenced and analyzed using parsimony. Both analyses did not yield monophyletic groups of species corresponding to Phoradendron and Dendrophthora, hence the single character that distinguishes them (anther locule number) appears to be weak justification for maintaining separate genera. Themes common to the above studies are that viscaceous mistletoes show increased rates of nucleotide substitution (Nickrent and Starr 1994) and that classifications based upon morphological features are often incongruent with those derived from genetic data.
D. Zygophyllales Takhtajan (1997). Krameriaceae Dumort.,
nom. cons. (1829).
Krameriaceae is a monotypic New World family with 17 species
of hemiparasitic perennial herbs and shrubs. The exact position
of the family among angiosperms is currently uncertain. Based
upon floral morphological and wood anatomical evidence, all modern
classifications (Cronquist 1988, Takhtajan 1997, Thorne 1992)
ally the family with Polygalales or Vochysiales. Despite this,
molecular analyses using rbcL (Chase et al. 1993, Sheahan
and Chase 1996), recover a sister relationship between Krameriaceae
and Zygophyllaceae, not with Polygalaceae which is itself sister
to the legumes. Nuclear SSU rDNA analyses (Soltis et al. 1997)
also allied Polygalaceae with legumes and apart from Zygophyllaceae
(Krameria was not included in that study). The APG (1998)
classification reflects these more recent concepts by placing
Krameriaceae in an unresolved position at the base of the rosids
along with Zygophyllaceae, Geraniales, and eight other families.
The recent three-gene analysis (Soltis et al. 2000), which included
560 angiosperms, placed Krameria with Guaiacum (100%
jackknife support) and this clade received 77% jackknife support
for its position within the eurosid I clade. Although there is
little support from morphological characters for a relationship
between Krameria and Zygophyllaceae, the molecular data
are clear, thus Krameriaceae is here classified in Zygophyllales.
E. Boraginales Dumort. (1829). Lennoaceae Solms, nom. cons. (1870). Lennoaceae, a small family of fleshy holoparasitic perennial herbs found in the deserts of North and South America, is composed of five species in two genera (Lennoa and Pholisma). Most modern classifications show a relationship with Boraginaceae; in fact, the APG (1998) classification includes Lennoaceae within that family. The circumscriptions of the orders Solanales, Lamiales and Boraginales have received varying interpretations, thus a variety of different classifications have resulted. The APG system placed Boraginaceae at the base of the euasterid I clade, apart from Lamiales and Solanales. Although higher-level classifications of these sympetalous dicots may change, it is likely that the association between Lennoaceae and Boraginaceae will remain. Recent molecular work using sequences from several genes (Smith et al. 2000) showed a relationship between Lennoaceae and subfamily Ehretioideae of Boraginaceae. Because this subfamily contains host plants of Lennoaceae, the authors suggest a possible case of adelphoparasitism, i.e. where a parasite is an evolutionary derivative of the host.
F. Solanales Dumort. (1829). Convolvulaceae Juss., nom. cons. (1789). The genus Cuscuta is composed of 160 species of hemiparasitic or holoparasitic vines. There is little doubt that these parasites are most closely related to Convolvulaceae yet disagreement exists as to whether Cuscuta should reside in this family or in its own (Cuscutaceae). The separate familial status is reflected in the classifications of Cronquist (1988), Takhtajan (1997), and Dahlgren (1983), whereas inclusion in Convolvulaceae is followed by Thorne (1992) and the APG (1998) classification. Indeed, molecular data appear to be conflicting on this topic. Mitochondrial gene and intron sequences revealed a sister relationship of Cuscuta and members of Convolvulaceae (McNeal and dePamphilis 2000), whereas analyses using four chloroplast gene regions resulted in Cuscuta being nested within Convolvulaceae (Stefanovic and Olmstead 2000). It is likely that resolution of this incongruence will require sampling the same suite of taxa for each gene, inclusion of a nuclear gene, and application of the same analytical methods to separate and combined data sets.
G. Lamiales Bromhead (1838). Scrophulariaceae Juss., nom. cons. (1789) and Orobanchaceae Vent., nom. cons. (1799). Scrophulariaceae and Orobanchaceae, as traditionally defined, include the greatest number of genera (85) and species (ca. 1600) of any parasitic flowering plant group. The former family contains many hemiparasites and the latter holoparasites, several of which are economically important pathogens of crops (e.g. Striga and Orobanche). The circumscription of these families is currently an area of active research and continues to generate controversy. For a synopsis of the problems surrounding classification of Scrophulariaceae, see Olmstead and Reeves (1995). A number of families, whose original circumscriptions date to the time of Bentham and Hooker (Bentham 1876), are known to be related to Scrophulariaceae, such as Globulariaceae, Lentibulariaceae, Martyniaceae, Myoporaceae, Orobanchaceae, and Pedaliaceae (Wageniz 1992). Moreover, clarifying relationships within Scrophulariaceae ultimately requires addressing the limits of other large families such as Acanthaceae, Bignoniaceae, Gesneriaceae, Lamiaceae, and Verbenaceae. While resolving some of these problems, molecular studies have also introduced additional hypotheses that, if fully implemented, will require major familial revisions. Analyses of the plastid genes rbcL and ndhF for 32 asterid genera (including nine Scrophulariaceae) showed that Scrophulariaceae was polyphyletic (Olmstead and Reeves 1995). Two major clades were identified, "scroph I" and "scroph II." The former is being referred to as Scrophulariaceae s. str. by the APG because the clade contains Scrophularia, the type of the oldest name. The scroph II clade, which contained Antirrhinum, Digitalis, Penstemon, Plantago, and Veronica was named Veronicaceae. Although no parasitic Scrophulariaceae or Orobanchaceae were included in the study by Olmstead and Reeves (1995), later studies (dePamphilis et al. 1997, Nickrent et al. 1998, Wolfe and dePamphilis 1998, Young et al. 1999) found these parasites to be monophyletic. The close relationship between the hemiparasitic (rhinanthoid) Scrophulariaceae and holoparasitic Orobanchaceae was confirmed and showed that holoparasitism had evolved more than once in the family. The recognition of one family helped solve the problem associated with several "transitional genera" (e.g. Harveya and Hyobanche) that had previously been classified in either Scrophulariaceae or Orobanchaceae. The most recent study, combining sequences from three plastid genes (rbcL, ndhF and rps2), recovered essentially the same three clades discussed above (Olmstead et al. 2000). The concept of one family (Orobanchaceae s. lat.) that encompasses the parasites was accepted by the APG group (1998) and was adopted in a recent plant systematics textbook (Judd et al. 1999). Additional discussion of this issue can be found in Olmstead et al. (2000), but it does not appear the issue is fully settled. Opponents to these new ideas mention that the revision is based entirely on chloroplast genes and that additional data from the other two genomes is required for confirmation. Specifically, it is worth noting that nuclear ITS rDNA sequence data are in conflict with the cpDNA gene trees in that they support a monophyletic Scrophulariaceae (A. Wolfe, pers. comm.).
H. Balanophorales Dumort. (1829). Balanophoraceae Rich. (1822). Balanophoraceae are succulent, squamate (with scale leaves or bracts) holoparasites whose haustorial system is either rhizomatous or massive and tuberous. Ten of the 17 genera are monospecific and all but Balanophora have four species or less. These rare tropical plants are seldom encountered, even by experienced botanists. The presence of numerous small genera on old landmasses (e.g. Madagascar, South Africa, South America, New Guinea, New Caledonia, etc.) suggests that these lineages are ancient. Because of extensive reductions, modifications, and losses of morphological features, classification of Balanophoraceae has been problematic. Indeed, some of the most reduced flowers known among angiosperms are found in this family, e.g. the pistillate flower of Balanophora is only one mm long. It is thus not surprising that tremendous differences of opinion exist as to which nonparasitic group is most closely related to these parasites. This is reflected in the classification systems of Cronquist and Takhtajan which are in most respects quite similar. Following the embryological work of Fagerlind (1948), Cronquist (1988) placed Balanophoraceae within Santalales (Rosidae). Although admitting that any similarities between the two groups could be due to convergence, a shared ancestor with Olacaceae was considered plausible. In sharp contrast to these ideas, Takhtajan (1997) placed Balanophorales in Magnoliidae, citing a common origin with Cynomoriaceae, Hydnoraceae and Rafflesiaceae. The position of Balanophoraceae was one of the changes seen between the phylogenetic systems of Thorne published in 1992 and 1999. The earlier system was similar to that of Cronquist in that Balanophoraceae were allied with Santalales in Santalanae, but were later elevated to equal rank (Balanophoranae). The APG (1998) classification was noncommittal by placing Balanophoraceae in the "Families of Uncertain Position" category. It is no exaggeration to state that elucidating the position of Balanophoraceae represents one of the truly difficult challenges remaining in angiosperm phylogenetics.
Several factors are important to consider when discussing the use of molecular data to address phylogenetic questions in Balanophoraceae. These plants lack rbcL and many of the other plastid genes commonly used for phylogenetic inference in green plants (Nickrent et al. 1997a, Nickrent et al. 1997b), thus compromising the ability to integrate these holoparasites into such data matrices. For this reason, nuclear rDNA was chosen for phylogenetic studies of these and other parasitic plants (Nickrent and Franchina 1990). These initial studies uncovered yet another phenomenon that can potentially confound phylogenetic analysis. Nuclear SSU rDNA sequences from Balanophoraceae (plus Hydnoraceae and Rafflesiaceae) show rates of nucleotide substitution elevated three times over those measured in green plants (Nickrent and Starr 1994). When these highly divergent sequences are analyzed along with less divergent ones, artifacts such as long-branch attraction (Felsenstein 1978) can occur.
Despite the above mentioned limitations, SSU rDNA sequences from ten genera of Balanophoraceae have been obtained. Substitution rates for some taxa are lower, thus long-branch artifacts are not expected to affect phylogenetic reconstruction. A phylogenetic tree showing intrafamilial relationships has been published (Nickrent and Duff 1996); however, this tree did not contain any other ingroup (nonparasitic) flowering plants. In large-scale studies of angiosperm phylogeny, it has been shown that combining data (in this case SSU rDNA, rbcL and atpB) results in shorter computer run times and greater resolution of relationships (Soltis et al. 1998). That data set contained 190 angiosperms, but has since been augmented and now contains 545 sequences. The tree given as Fig. 1 in the APG (1998) publication was derived from analysis of this matrix which was kindly made available by D. Soltis. The data set was modified by the deletion of most monocots and by the addition of ten Balanophoraceae SSU rDNA sequences, thus yielding a matrix of 4631 characters (nucleotides) by 463 ingroup taxa and seven outgroup gymnosperms. The strategy here is to stabilize the topology of the global angiosperm tree by using more nucleotide characters. The rbcL and atpB genes were coded as missing for the holoparasites, thus their position was determined solely by the rDNA gene. Although the heuristic search did not go to completion, the resulting consensus tree was very similar to the one reported by the APG (1998). In general agreement with the classification of Fagerlind (1948) and Cronquist (1981, 1988), Balanophoraceae emerged as monophyletic and sister to Santalales as part of the unresolved core eudicot clade containing Caryophyllales. Cynomorium was not allied with this clade (see below). These results are certainly preliminary and will require further testing using other genes, particularly those from the mitochondrion.
I. Cynomoriales Burnett (1835). Cynomoriaceae (Schott & Endl.) Lindl., nom. cons. (1833). Cynomorium is a root holoparasite with one (possibly two) species that occurs in dry areas surrounding the Mediterranean Sea to central Asia. Owing to general similarities in habit and inflorescence morphology, the genus has most often been placed within or near Balanophoraceae. Some modern classification systems relegate the genus to a separate family, Cynomoriaceae, e.g. Takhtajan (1997), Thorne (1992), and Dahlgren (1983) whereas Cronquist (1981) maintained it within Balanophoraceae. A number of morphological, embryological, and karyological differences can be listed that distinguish the two families (Leonard 1986, Pazy et al. 1996, Teryokhin et al. 1975). For this reason, it is of interest to use molecular characters to determine whether Cynomorium is part of Balanophoraceae or a distinct lineage and, if the latter is true, to identify its nearest nonparasitic relatives. A nuclear SSU rDNA sequence for Cynomorium was included in the three-gene matrix described above. Cynomorium was not part of the Balanophoraceae clade but associated with the Saxifragales clade (including Grossulariaceae, Saxifragaceae, and Haloragaceae). When SSU rDNA sequences are analyzed separately, Cynomorium differs from New World members of Balanophoraceae by ca. 140 steps and from Old World taxa by ca. 200 steps. Thus, the distinctiveness of this family is strongly supported by molecular data whereas its position within the global angiosperm phylogeny will require additional analyses for confirmation.
I. Rafflesiales Oliv. (1895). Apodanthaceae (R. Br.) Tiegh. ex Takht. (1987), Cytinaceae (Brongn.) A. Rich. (1824), Mitrastemonaceae Makino, nom. cons. (1911), and Rafflesiaceae Dumort., nom. cons. (1829). This group (here considered an order) comprises four families, and includes some of the most remarkable evolutionary productions among all flowering plants. At one extreme is Pilostyles (Apodanthaceae) whose flower is less than a centimeter long. At the other extreme is Rafflesia (Rafflesiaceae), the "queen of the parasites," whose flowers may measure over one meter in diameter. The vegetative parts of these plants are entirely haustorial, i.e. a reduced endophyte that grows within the host root or stem. Only the flowers or inflorescences are present outside the host tissue.
Distinctive floral morphology as well as differences in the ovules and seeds (Bouman and Meijer 1994) support the recognition of four families of Rafflesiales: 1) the "small-flowered" taxa (Apodanthaceae) including Apodanthes and Pilostyles; 2) the "large-flowered taxa" (Rafflesiaceae sensu stricto) including Rafflesia, Rhizanthes, and Sapria; 3) the inflorescence-forming taxa (Cytinaceae), including Cytinus and Bdallophyton, and 4) Mitrastema (Mitrastemonaceae), the only member of the order possessing a superior ovary. Features shared by these genera are a tendency to have unisexual flowers (plants dioecious), the presence of a central column, and a tendency toward parietal placentation. The morphological features that remain have been so altered over the course of their evolution as to confound comparison with more typical plants. Cronquist (1981) discussed the affinities of Rafflesiales with Santalales, noting similarities of the endophyte of Viscaceae. Although acknowledging that these structures evolved in parallel, curiously he suggested that such parallelism is a reflection of relationship. As discussed above for Hydnoraceae, Takhtajan (1997) derived Rafflesiales from Aristolochiaceae, supporting this position with results of work from 19th century botanists as well as Bouman and Meijer (1994). That classification placed Rafflesiales in subclass Magnoliidae, not Rosidae. The magnoliid affinity was also reflected in the classifications of Thorne (1992) and Dahlgren (1983). With regard to classification of parasitic plants, Rafflesiales may be the most enigmatic The APG (1998) classification placed Rafflesiaceae s. lat. with a group including Hydnoraceae and nine autotrophic magnoliid families at the base of the angiosperm tree (hence unresolved).
No molecular phylogeny of Rafflesiales has yet been published. As was done with Balanophoraceae, a data matrix was assembled using SSU rDNA, rbcL, and atpB for 467 plants (7 outgroup gymnosperms, Hydnora and Prosopanche, 452 autotrophic angiosperms, and six genera of Rafflesiales). Nuclear SSU rDNA sequences have yet to be obtained from Apodanthaceae. Branch swapping did not go to completion, hence the resulting consensus tree was not optimal and should be considered only preliminary evidence. Rafflesiales emerged as monophyletic from within the Malvales clade. This is in agreement with results obtained by the dePamphilis lab (reported in Milius (1999)) using a multigene data set that included mitochondrial cox1 and atp1 (Barkman et al. 2000). As discussed above, Hydnoraceae is allied with Aristolochiaceae, hence is not closely related to Rafflesiales. A phylogenetic relationship between Hydnoraceae and Rafflesiaceae s. lat. has generally been assumed in nearly every published angiosperm classification. Because of this, certain character discontinuities, such as monosulcate pollen in Hydnora but triaperturate pollen in Rafflesiaceae, compelled Cronquist (1981) to resort to explanations involving reversions to a more primitive condition in the former genus. If Hydnoraceae and Rafflesiaceae truly have different phylogenetic origins, as appears to be the case from molecular evidence, these discontinuities are fully explainable.
Further research on the phylogenetic relationships of Rafflesiales is currently underway in a collaboration involving A. Blarer, Y.-L. Qiu, D. Soltis, and D. Nickrent. The five-gene data set (including mitochondrial atp1 and matR) was used to investigate phylogenetic relationships of Rafflesiales (Blarer et al. 2000). Assuming Berlinianche is synonymous with Pilostyles (as proposed by Bouman (1994)), complete generic sampling was achieved and thus the position of Apodanthaceae relative the other families of the order could be addressed. In this analysis, Rafflesiales emerges as monophyletic and separate from Hydnorales. Clades corresponding to the four families of Rafflesiales were recovered. As with the three gene study discussed above, the Rafflesiales clade grouped within the eudicots (near Brassica). Unfortunately, only 12 eudicot sequences were included in this analysis owing to paucity of mitochondrial gene sequences, hence further resolution awaits acquisition of these data. It is of interest that both Brassicaceae and Malvales are components of the eurosid II clade as shown in the APG (1998) classification, thus this result merits further investigation.
Summary of Parasitic Plant Phylogeny
As stated in the introduction, determining the phylogenic
relationships among parasitic plants has remained as challenging
for modern systematists using DNA sequences as for botanists working
on these groups a century ago. From the above discussions, it
now appears that parasitism has arisen independently ten times:
Balanophoraceae, Convolvulaceae, Cynomoriaceae, Hydnoraceae,
Krameriaceae,
Lauraceae, Lennoaceae, Santalales, Orobanchaceae s. lat., and
Rafflesiales. The monophyletic status of Balanophoraceae and
Rafflesiales
are most open to question and require further study for confirmation.
Two families, Convolvulaceae and Orobanchaceae s. lat. have both
hemiparasitic and holoparasitic members, thus present unique
opportunities
for studying the physiological and genetic changes that evolve
with increased dependence upon the host. Similarly, the origin
of parasitism can be better understood by studying families where
both parasitic and autotrophic members occur, e.g. Olacaceae and
the "edges" of the newly defined Orobanchaceae (e.g.
Lindenbergia). Analyses of molecular data have helped answer
long-standing questions in parasitic plant phylogeny. It is likely
that the last problems to be resolved among all major flowering
plant groups will be those surrounding parasitic angiosperms.
APG. 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531-553.
Ashworth, V. E. T. M. 2000a. Phylogenetic relationships in Phoradendreae (Viscaceae) inferred from three regions of the nuclear ribosomal cistron. I. Major lineages and parphyly of Phoradendron. Syst. Bot. 25: 349-370.
Ashworth, V. E. T. M. 2000b. Utility of trnL-trnF noncoding sequences of chloroplast DNA in inferring phylogenetic relationships in Phoradendreae (Viscaceae): presence of a homoplastic inversion in the intergenic spacer. Pl. Syst. Evol.
Baas, P. 1982. Leaf anatomy and classification of the Olacaceae, Octoknema and Erythropalum. Allertonia 3: 155-210.
Barkman, T. J., J. R. McNeal, N. D. Young, and C. W. dePamphilis. 2000. Multiple origins of parasitism within angiosperms. Amer. J. Bot. [Suppl.] 87: 112.
Barlow, B. A. 1964. Classification of the Loranthaceae and Viscaceae. Proc. Linn. Soc. N. S. W. 89: 268-272.
Bentham, G. B. 1876. Scrophulariaceae in G. Bentham and J. D. Hooker, eds. Genera Plantarum. Reeves, London.
Bhandari, N. N., and S. C. A. Vohra. 1983. Embryology and affinities of Viscaceae. Pages 69-86 in M. Calder and P. Bernhardt, eds. The Biology of Mistletoes. Academic Press, New York.
Blarer, A., D. L. Nickrent, H. Bänziger, P. K. Endress, and Y.-L. Qiu. 2000. Phylogentic relationships among genera of the parasitic familiy Rafflesiaceae s.l. based on nuclear ITS and SSU rDNA, mitochondrial LSU and SSU rDNA, atp1, and matR sequences. Amer. J. Bot. [Suppl.] 87: 171.
Bouman, F., and W. Meijer. 1994. Comparative structure of ovules and seeds in Rafflesiaceae. Pl. Syst. Evol. 193: 187-212.
Cartellieri, E. 1928. Das Haustorium von Cassytha pubescens R. Br. Planta 6: 162-182.
Chase, M. W., D. E. Soltis, R. G. Olmstead, et al. 1993. Phylogenetics of seed plants: An analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528-580.
Cocucci, A. E. 1975. Estudios en el género Prosopanche (Hydnoraceae) II. Organización de la flor. Kurtziana 8: 7-15.
Cocucci, A. E. 1976. Estudios en el género Prosopanche (Hydnoraceae) III. Embriología. Kurtziana 9: 19-39.
Cronquist, A. 1981. An integrated system of classification of flowering plants. Columbia Univ. Press, New York, NY.
Cronquist, A. 1988. The evolution and classification of flowering plants. New York Botanical Gardens, New York, NY.
Dahlgren, R. 1983. General aspects of angiosperm evolution and macrosystematics. Nordic J. Bot. 3: 119-149.
Danser, B. H. 1933. A new system for the genera of Loranthaceae-Loranthoideae, with a nomenclator for the Old World species of this subfamily. Verhandelingen der Koninklijke Akademie van Wetenschappen te Amsterdam Afdeeling Natuurkunde Sect. 2, 29: 1-128.
dePamphilis, C. W., N. D. Young, and A. D. Wolfe. 1997. Evolution of plastid gene rps2 in a lineage of hemiparasitic and holoparasitic plants: many losses of photosynthesis and complex patterns of rate variation. Proc. Nat. Acad. Sci. USA 94: 7367-7372.
Donoghue, M. J., and J. A. Doyle, eds. 1989. Phylogenetic analysis of angiosperms and the relationships of Hamamelidae. Clarendon Press, Oxford.
Fagerlind, F. 1948. Beiträge zür Kenntnis der Gynäceummorphologie und Phylogenie der Santalales-Familien. Sv. Bot. Tidskr. 42: 195-229.
Felsenstein, J. 1978. Cases in which parsimony or compatibility will be positively misleading. Syst. Zool. 27: 401-410.
Heide-Jørgensen, H. S. 1987. Changes in cuticle structure during development and attachment of the upper haustorium of Cuscuta L., Cassytha L., and Viscum L. Pages 319-334 in H. C. Weber and W. Forstreuter, eds. Parasitic Flowering Plants. Philipps-Universität, Marburg, Germany.
Heo, K., H. Van der Werff, and H. Tobe. 1998. Embryology and relationships of Lauraceae (Laurales). Bot. J. Linn. Soc. 126: 295-322.
Hoot, S. B., A. Culham, and P. R. Crane. 1995. The utility of atpB gene sequences in resolving phylogenetic relationships: comparison with rbcL and 18S ribosomal DNA sequences in the Lardizabalaceae. Ann. Missouri Bot. Gard. 82: 194-207.
Hoot, S. B., S. Magallón, and P. R. Crane. 1999. Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Ann. Missouri Bot. Gard. 86: 1-32.
Judd, W. S., C. S. Campbell, E. A. Kellogg, and P. F. Stevens. 1999. Plant Systematics: A Phylogenetic Approach. Sinauer Associates, Inc., Sunderland, MA.
Kuijt, J. 1968. Mutual affinities of Santalalean families. Brittonia 20: 136-147.
Kuijt, J. 1969. The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA.
Kuijt, J. 1988. Monograph of Eremolepidaceae. Systematic Botany Monographs 18: 1-60.
Leonard, J. 1986. Observation sur le genre Cynomorium L. en Asie (Cynomoriaceae). Bulletin du Jardin Botanique de L'Etat Bruxelles 56: 301-304.
McNeal, J. R., and C. W. dePamphilis. 2000. Origin and molecular systematics of the parasitic plant genus Cuscuta (dodder). Amer. J. Bot. [Suppl.] 87: 144.
Milius, S. 1999. The science of big, weird flowers. Science News 156: 172-174.
Molvray, M., P. J. Kores, and M. W. Chase. 1999. Phylogenetic relationships within Korthalsella (Viscaceae) based on nuclear ITS and plastid trnL-F sequence data. Amer. J. Bot. 86: 249-260.
Musselman, L. J., and J. H. Visser. 1986. The strangest plant in the world! Veld and Flora 71: 109-111.
Nayar, B. K., and P. N. Nayar. 1952. On the range of the hosts of Cassytha filiformis Linn. Sci. and Cult 17: 383-384.
Nickrent, D. L. 1996. Molecular Systematics. Pages 155-170 in F. G. Hawksworth and D. Wiens, eds. Dwarf mistletoes: Biology, Pathology, and Systematics. USDA Forest Service Agricultural Handbook 709, Washington DC.
Nickrent, D. L., and R. J. Duff. 1996. Molecular studies of parasitic plants using ribosomal RNA. Pages 28-52 in M. T. Moreno, J. I. Cubero, D. Berner, D. Joel, L. J. Musselman, and C. Parker, eds. Advances in Parasitic Plant Research. Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, Spain.
Nickrent, D. L., R. J. Duff, A. E. Colwell, A. D. Wolfe, N. D. Young, K. E. Steiner, and C. W. dePamphilis. 1998. Molecular phylogenetic and evolutionary studies of parasitic plants. Pages 211-241 in D. E. Soltis, P. S. Soltis, and J. J. Doyle, eds. Molecular Systematics of Plants II. DNA Sequencing. Kluwer Academic Publishers, Boston, MA.
Nickrent, D. L., R. J. Duff, and D. A. M. Konings. 1997a. Structural analyses of plastid-derived 16S rRNAs in holoparasitic angiosperms. Plant Mol. Biol. 34: 731-743.
Nickrent, D. L., and C. R. Franchina. 1990. Phylogenetic relationships of the Santalales and relatives. J. Mol. Evol. 31: 294-301.
Nickrent, D. L., and V. Malecot. 2000. Phylogenetic relationships of Santalales based on nuclear small-subunit (18S) rDNA and rbcL with special reference to Olacaceae. Unpublished Abstract, Botany 2000 meetings, August 6-10, Portland, Oregon USA.
Nickrent, D. L., Y. Ouyang, R. J. Duff, and C. W. dePamphilis. 1997b. Do nonasterid holoparasitic flowering plants have plastid genomes? Plant Mol. Biol. 34: 717-729.
Nickrent, D. L., K. P. Schuette, and E. M. Starr. 1994. A molecular phylogeny of Arceuthobium based upon rDNA internal transcribed spacer sequences. Amer. J. Bot. 81: 1149-1160.
Nickrent, D. L., and D. E. Soltis. 1995. A comparison of angiosperm phylogenies based upon complete 18S rDNA and rbcL sequences. Annals of the Missouri Botanical Garden 82: 208-234.
Nickrent, D. L., and E. M. Starr. 1994. High rates of nucleotide substitution in nuclear small-subunit (18S) rDNA from holoparasitic flowering plants. J. Mol. Evol. 39: 62-70.
Olmstead, R. G., C. W. dePamphilis, A. D. Wolfe, N. D. Young, W. J. Elisens, and P. J. Reeves. 2000. Disintegration of the Scrophulariaceae. Amer. J. Bot. 87: x-y.
Olmstead, R. G., and P. A. Reeves. 1995. Evidence for the polyphyly of the Scrophulariaceae based on chloroplast rbcL and ndhF sequences. Annals of the Missouri Botanical Garden 82: 176-193.
Parkinson, C. L., K. L. Adams, and J. D. Palmer. 1999. Multigene analyses identify the three earliest lineages of extant flowering plants. Curr. Biol. 9: 1485-1488.
Pazy, B., U. Plitmann, and O. Cohen. 1996. Bimodal karyotype in Cynomorium coccineum L. and its systematic implications. J. Linn. Soc. London 120: 279-281.
Pilger, R. 1935. Santalaceae. Pages 52-91 in A. Engler and K. Prantl, eds. Die Naturlichen Pflanzen Familien, Leipzig.
Polhill, R., and D. Wiens. 1998. Mistletoes of Africa. The Royal Botanic Gardens Kew, Richmond-Surrey, UK.
Qiu, Y., Y. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zanis, E. A. Zimmer, Z. Chen, V. Savolainen, and M. W. Chase. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404-409.
Rao, P. R. M. 1980. Seed and fruit anatomy of Cassytha filiformis L. with comments on its systematic position. Israel J. Bot. 28: 44-49.
Renner, S. S., and A. Chanderbali. 2000. What is the relationship among Hernandiaceae, Lauraceae, and Monimiaceae, and why is this question so difficult to answer? Int. J. Plant Sci. 161:S109-S119.
Reveal, J. L. 1998. Indices nominum supragenericorum plantarum vascularium. Internet link HERE.
Rohwer, J. G. 2000. Toward a phylogenetic classification of the Lauraceae: Evidence from matK sequences. Syst. Bot. 25: 60-71.
Ronse Decraene, L. P., E. F. Smets, and P. Vanvinckenroye. 1998. Pseudodiplostemony, and its implications for the evolution of the androecium in the Caryophyllaceae. J. Plant Res. 111: 25-43.
Sastri, R. L. N. 1956. Embryo sac haustoria in Cassytha filiformis Linn. Curr. Sci. 25: 401-402.
Sastri, R. L. N. 1962. Studies in Lauraceae. III Embryology of Cassytha. Bot. Gaz. 123: 197-206.
Sheahan, M., and M. Chase. 1996. A phylogenetic analysis of Zygophyllaceae R Br based on morphological, anatomical and rbcL DNA sequence data. Bot. J. Linn. Soc. 122: 279-300.
Sleumer, H. O. 1984a. Olacaceae. Pages 1-29. Flora Malesiana I. The Hague.
Sleumer, H. O. 1984b. Olacaceae Monograph No 38. New York Botanical Garden, New York.
Smith, R. A., D. M. Ferguson, T. J. Barkman, and C. W. dePamphilis. 2000. Molecular phylogenetic evidence for the origin of Lennoaceae: A case of adelphoparasitism in the angiosperms? Amer. J. Bot. [Suppl.] 87: 158.
Solms-Laubach, H. 1894. Hydnoraceae. Pages 282-285 in A. Engler and K. Prantl, eds. Die Natürlichen Planzenfamilien, Part III, Leipzig.
Soltis, D. E., P. S. Soltis, M. W. Chase, et al.. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnaean Society 133:381-461.
Soltis, D. E., P. S. Soltis, M. E. Mort, M. W. Chase, V. Savolainen, S. B. Hoot, and C. M. Morton. 1998. Inferring complex phylogenies using parsimony: an empirical approach using three large DNA data sets for angiosperms. Syst. Biol. 47: 32-42.
Soltis, D. E., P. S. Soltis, D. L. Nickrent, et al.. 1997. Angiosperm phylogeny inferred from 18S ribosomal DNA sequences. Ann. Missouri Bot. Gard. 84: 1-49.
Soltis, P. S., D. E. Soltis, and M. W. Chase. 1999. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402: 402-407.
Stearn, W. T. 1972. Kunkeliella, a new genus of Santalaceae in the Canary Islands. Cuad. Bot. Canar. 16: 11-26.
Stefanovic, S., and R. G. Olmstead. 2000. Molecular systematics of Convolvulaceae inferred from cpDNA sequences. Amer. J. Bot. [Suppl.] 87: 160.
Takhtajan, A. 1997. Diversity and classification of flowering plants. Columbia University Press, New York.
Teryokhin, E. S., M. S. Yakovlev, and Z. I. Nikitcheva. 1975. Development of microsporangium, pollen grains, ovule and embryo sac in Cynomorium songaricum Rupr. (Cynomoriaceae). Botanicheskii Zhurnal 60: 153-162.
Thorne, R. F. 1992. An updated phylogenetic classification of the flowering plants. Aliso 13: 365-389.
van der Werff, H., and H. G. Richter. 1996. Toward an improved classification of Lauraceae. Annals of the Missouri Botanical Garden 83: 409-418.
Wageniz, G. 1992. The Asteridae: evolution of a concept and its present status. Ann. Missouri Bot. Gard. 79: 209-217.
Weber, J. L. 1981. A taxonomic revision of Cassytha (Lauraceae) in Australia. J. Adelaide Bot. Gard. 3: 187-262.
Werth, C., W. P. Pusateri, W. H. Eshbaugh, and T. K. Wilson. 1979. Field observations on the natural history of Cassytha filiformis L. (Lauraceae) in the Bahamas. Pages 94-102 in L. J. Musselman, A. D. Worsham, and R. E. Eplee, eds. Second International Symposium on Parasitic Weeds. North Carolina State University, Raleigh, N.C.
Wiens, D., and B. A. Barlow. 1971. The cytogeography and relationships of the viscaceous and eremolepidaceous mistletoes. Taxon 20: 313-332.
Wolfe, A. D., and C. W. dePamphilis. 1998. The effect of relaxed functional constraints on the photosynthetic gene rbcL in photosynthetic and nonphotosynthetic parasitic plants. Mol. Biol. Evol. 15: 1243-1258.
Young, N. D., K. E. Steiner, and C. W. dePamphilis. 1999. The evolution of parasitism in Scrophulariaceae/ Orobanchaceae: Plastid gene sequences refute an evolutionary transition series. Annals of the Missouri Botanical Garden 86: 876-893.
Figure 1. Proposed phylogenetic relationships among angiosperms with incidences of haustorial parasitism indicated on the phylogram (ten evolutionary origins). The basic structure of the tree is based upon one reported by the APG and Soltis et al. with modifications (particularly for the monosulcate groups) from Soltis et al. , Qiu et al. and Parkinson et al. . Branch lengths shown on this figure have no relationship to patristic distance. The acronym "ITA" (at asterisk *) refers to Illiciaceae, Trimeniaceae, and Austrobaileyaceae. See text for explanations of the positions of the parasite orders and families.
SIUC / College of Science / Parasitic Plant Connection
URL: http://www.parasiticplants.siu.edu/Chapter3.html
Last updated: 22-Mar-11 / dln