The following is the original English version of Chapter 2 that was translated into Spanish by Pilar Catalán with assistance from Miguel A. García.The Spanish citation is:Nickrent, D. L. 2002. Plantas parásitas en el mundo. Capitulo 2, pp. 7-27 in J. A. López-Sáez, P. Catalán and L. Sáez [eds.], Plantas Parásitas de la Península Ibérica e Islas Baleares. Mundi-Prensa, Madrid.The English citation is:Nickrent, D. L. 2002. . Parasitic Plants of the World. Chapter 2, pp. 7-27 in J. A. López-Sáez, P. Catalán and L. Sáez [eds.], Parasitic Plants of the Iberian Peninsula and Balearic Islands. Mundi-Prensa, Madrid. |
1. General Introduction
Just as insectivorous plants have captured the imagination of humans for many generations, parasitic plants have also been the subject of much curiosity since ancient times. What these two groups of plants have in common is that they have both evolved nutritional modes that are strikingly different than what is typically perceived to be "normal" for a green plant, i.e. photosynthetic, motionless, and oblivious to other organisms around them. One cannot help but marvel that evolution has produced insectivorous plants that "turn the tables" (specifically, the dinner tables!) on animals by actively trapping invertebrates (insects) and digesting them, thereby releasing nitrogenous compounds that are otherwise limited in the plant's environment. Similarly, parasitic plants represent another complex nutritional mode that has evolved independently in at least nine lineages (see next chapter), but in this case the parasite's alternate source of food is from another plant.
2. Nonparasitic Associations
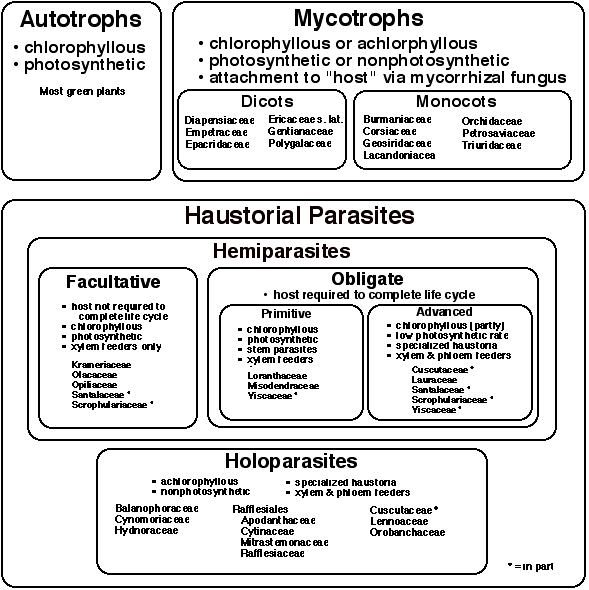
To place parasitism into its proper context, it is useful to look at the full spectrum of trophic modes present within all flowering plants (Fig. 1). The vast majority of green plants (angiosperms) that are encountered daily are autotrophic, i.e. they produce all their own food via photosynthesis. In contrast, there are a significant number of plants that have adopted a heterotrophic mode whereby all or some of their carbohydrates are obtained from another organism. One can categorize heterotrophs into two major categories: mycotrophs and haustorial parasites (Furman and Trappe 1971) (Fig. 1). Mycotrophs, sometimes mistakenly referred to as saprophytes (only fungi are truly saprophytic), obtain carbohydrates and other nutrients by parasitizing a mycorrhizal fungus. Often the mycorrhizae are also associated with the roots of photosynthetic trees, hence the mycotroph can be thought to indirectly parasitize the tree. Mycotrophs can be found in a number of monocot and dicot families (Fig. 1) representing over 400 species. One frequently encountered mycotroph is Monotropa whose ghostly white appearance suggests the fact that chlorophyll is lacking and that it receives its nutrition in a heterotrophic fashion (see Chapter 6 by L. Villar). Because of its nonphotosynthetic nature, Monotropa is often misinterpreted as a parasitic plant, and indeed there is some degree of penetration of the host roots by Monotropa (Bjorkmann 1960), hence it may be considered an epiparasite.
As discussed in the first chapter of his book "The Biology of Parasitic Flowering Plants," Kuijt (1969) describes a number of other associations between plants that have been considered parasitic. These include an Opuntia cactus growing from the stem of Idria, a Passiflora growing within Euonymus, and stem and root grafts that occur commonly among different tree species. At least for the latter examples, there is clear documentation of the movement of nutrients between the two partners, thus making the definition of parasitism an exercise in semantics. It is for this reason that the term "haustorial parasite" has been used in this chapter to restrict usage to those plants that form modified roots called haustoria that affect the morphological and physiological connection to another plant. With the possible exception of Hyobanche which can form secondary haustoria from scale leaves (Kuijt et al. 1978, Visser et al. 1978), essentially all haustoria are modified roots.
Haustorial parasitism appears to have evolved only in flowering plants (dicots), however, the case of Parasitaxus ustus (Viellard) de Laubenfels, a rare gymnosperm of the family Podocarpaceae, must also be considered. This shrub or small tree has fleshy, deep wine red to purple scale leaves, and is found only in New Caledonia where it occurs in remote, densely forested areas. The plant lacks roots and is always found attached to the roots of Falcatifolium taxoides (Brongn. & Gris) de Laubenfels, which is also a member of Podocarpaceae. There is debate about the parasitic status of this plant because a typical haustorium is not formed but the xylem-to-xylem connections resemble a root graft (Köpke et al. 1981). More recent work (Woltz et al. 1994) indicates that the Parasitaxus haustorium penetrates to the cambium of the host and that both plants are infected by a mycelial endophyte that links them in a symbiotic relationship. Thus, it seems that this gymnosperm is not a haustorial parasite but a mycotroph.
3. Nutritional Modes in Parasitic Plants
One can categorize parasitic plants according to their evolutionary relationships (next chapter) or according to their nutritional mode. Among the various unrelated families of parasitic plants, two basic types of parasitism exist: hemiparasites and holoparasites (Fig. 1). Hemiparasites are chlorophyllous and photosynthetic (at least during some portion of the life cycle) yet they obtain water and nutrients via haustorial connections to the host plant. Hemiparasites can be further divided into two types, facultative and obligate, depending upon their degree of dependence upon the host. Facultative hemiparasites do not require a host to complete their life cycle but are photosynthetic and, when presented with host roots, invariably form haustorial connections. When attached to host roots, these parasites extract water and dissolved minerals via direct, cell-to-cell connections to the xylem. Facultative hemiparasites can be found in several root-parasitic families, e.g. Olacaceae, Opiliaceae, Santalaceae (Santalales), Krameriaceae (Fabales), and Scrophulariaceae (Lamiales). Experimental work has documented the fact that parasitic Scrophulariaceae from Europe (e.g. Bartsia, Euphrasia, Melampyrum, Odontites, Pedicularis and Rhinanthus) and North America (Agalinis, Dasistoma, Macranthera, and Seymeria) can be grown to flowering and fruiting in the absence of host plants (Weber 1981, Mann and Musselman1981). Although some genera in Olacaceae are clearly facultative hemiparasites, others such as Olax may represent transitional states between facultative and obligate. For example, seedlings of O. phyllanthi first exist in a preparasitic state without developing haustoria but will succumb after six months if they do not attach to a host (Pate et al. 1990a, Pate et al. 1990b). To distinguish between the facultative and obligate conditions, it must be determined if the parasite is able to flower and fruit during the preparasitic stage. Plants grown from seed of Osyris alba (Sanalaceae) survived and flowered for several years in pots without hosts (Nickrent, pers. obs.), however, the cause of the eventual death of these potted plants was not determined.
Obligate hemiparasitism represents a further advancement in a continuum of parasitism types. In contrast to facultative hemiparasites, obligate hemiparasites must attach to a host plant to complete their life cycle. Among the obligate hemiparasites, one can differentiate two types, primitive and advanced (Fig. 1). The primitive type includes stem parasites of Loranthaceae, Misodendraceae and some Viscaceae. These plants are photosynthetic xylem feeders, but, being stem parasites, they cannot exist independent of the host plant. Possible exceptions to this may be the root parasites found in Loranthaceae (Atkinsonia, Gaiadendron, and Nuytsia) which, unlike other members of the family, do not form primary but only secondary (lateral) haustoria. For Atkinsonia (Menzies 1959), Gaiadendron (Kuijt 1963) and Nuytsia (Main 1947) seedlings can exist independent of a host plant for many months to at least a year. For Nuytsia, this phase is transitory, for seedlings will die within six to nine months if not provided a host plant (Main 1947). Interestingly, their life can be extended to at least three years with fertilization and hormone treatment (Grieve 1975). It is unlikely that any of these mistletoes are capable of independent existence for the long term because all lack root hairs that are essential for nutrient uptake (Hocking 1983). Six of the seven genera of Viscaceae can be categorized as primitive obligate hemiparasites, the exception being Arceuthobium whose adult shoots fix only about 30% of the carbon needed for growth (Hull and Leonard 1964). The advanced obligate hemiparasites attach not only to host xylem but also obtain host carbon via phloem connections. Concomitant with this nutritional mode is the loss of photosynthetic function, at least to some degree or during some stage of the life cycle. Examples of this nutritional mode include most species of Cuscuta (Convolvulaceae), Cassytha (Lauraceae), Phacellaria (Santalaceae), Striga gesnerioides (Scrophulariaceae), and Arceuthobium (Viscaceae).
The most extreme manifestation of the parasitic mode can be found among the holoparasites. These plants are totally achlorophyllous (or nearly so), nonphotosynthetic, and obtain all their water and nutrients from host xylem and phloem. Most holoparasites occur on host roots, however, some species of Cuscuta (e.g. C. europaea) are stem holoparasites that have lost RUBISCO, thylakoids, chlorophyll and light-dependent CO2 fixation (Machado and Zetsche 1990). Some members of Rafflesiales also occur as stem parasites (e.g. Apodanthes, Pilostyles, Rafflesia), but it is likely that these first become established on host roots and continued growth of the endophyte results in relocation to the stem (see Kuijt 1969, p. 207). Holoparasitism has evolved independently in at least seven lineages: Balanophoraceae, Cynomoriaceae, Hydnoraceae, Rafflesiales, Convolvulaceae, Lennoaceae and Scrophulariaceae (Fig. 1); however, the taxonomic circumscription and phylogenetic positions of these plants is currently undergoing intensive re-evaluation (see next chapter). Two families, Convolvulaceae (Cuscuta) and Scrophulariaceae (the latter in the broad sense, including Orobanchaceae) serve as models for studying the evolution of holoparasitism from hemiparasitism because both modes (and transitional forms) exist among particular species.
4. Numbers and Distributions of parasitic plants
Parasitic plants are never the dominant life form in an ecosystem, yet what can be said about their relative diversity? As shown in Table 1, there exists approximately 3900 species of haustorial parasitic plants, that is, just over 1% of all flowering plants. The total number of parasite species is not distributed evenly among the 18 families or 278 genera. Almost half the total can be attributed to Scrophulariaceae s. lat. and within this family, the genera Castilleja (200 spp.), Euphrasia (170 spp.), and Pedicularis (350 spp.) account for over 700 species. Other large genera (ca. 100 species or more) include Cuscuta, Amyema (Loranthaceae), and Viscum and Phoradendron (Viscaceae). Thus, 30% of all parasite species can be attributed to the above seven genera. These figures are of course dependent upon current taxonomic concepts. For example, the circumscription of Tapinanthus in the recent "Mistletoes of Africa" (Polhill and Wiens 1998) indicates the genus is composed of 30 species, reduced from previous estimates of nearly 250 species by broader species concepts and taxonomic transfers to other genera.
One can encounter parasitic plants in nearly every habitat type found throughout the world. For example, Pedicularis dasyantha occurs on the Svalbard archipelago at latitudes of more than 80° N (Musselman and Press 1995). At the opposite pole, Nanodea muscosa occurs in Tierra del Fuego Argentina at 55° S. Families such as Balanophoraceae, Rafflesiaceae, Mitrastemonaceae, Loranthaceae, and Olacaceae have significant numbers of genera and species in moist tropical habitats. Grassland and savannah ecosystems that receive less precipitation also harbor diverse parasite floras, particularly in the families Scrophulariaceae and Loranthaceae. Even in xeric habitats such as deserts, parasitic plants such as Hydnoraceae, Cynomoriaceae, Lennoaceae and Apodanthaceae can be found. It should be remembered that parasitic plants must have lower water potentials than their host plants to ensure the flow of water and nutrients moving through the haustorial connection. For many parasites, this is accomplished by maintaining higher transpiration rates than that of their host plants. This may be the reason that one does not encounter large numbers of stem parasitic Loranthaceae and Viscaceae in dense (hence dark) tropical rainforest conditions. Savannas, with their high solar incidence and numerous host trees, provide ideal situations for these mistletoes, and significant adaptive radiations in these families have taken place in such dry ecosystems in Central and South America, Africa and Australia.
In terms of overall numbers, the majority of parasitic plant species occur in ecosystems undisturbed by humans. In the minority are those parasite species that have adaptations to human disturbance and thereby increase in population size. In the case of Phoradendron leucarpum (= P. serotinum) in the Eastern United States, the patchy distribution of host trees in urban and park settings appears ideal for the spread and proliferation of this parasite. The heaviest concentration of this mistletoe was in residential areas of middle Tennessee, USA (McKinney and Hemmerly 1977) and this is likely the case throughout much of the distribution of this parasite. A patchy distribution of host trees (instead of dense forest stands) appears to limit and concentrate the movements of seed-dispersing birds such as waxwings (Skeate 1987), thus allowing the development of large parasite populations. Even-aged stands of coniferous species derived from large-scale replantings can support explosive increases in populations of Arceuthobium, particularly when these plantations are adjacent to infected old-growth trees (Hawksworth and Wiens 1996 p. 136). Fragmentation of Acacia woodlands in the Northern Territory of Australia and the movements of the mistletoe bird Dicaeum hirundinaceum apparently increases the incidences of parasitism by Amyema preissii (Lavorel et al. 1999). Striga and Orobanche each have species highly adapted to agricultural habitats. Indeed, it has been hypothesized that Striga hermonthica coevolved with wild relatives of Sorghum (Mohamed et al. 1996) and the current pathogenic strain of the species has only emerged following domestication of this crop (Kuiper 1997). The reproductive potential of these plants is high (given their numerous tiny seeds), hence large populations of susceptible crop species offer numerous opportunities for parasitism (Musselman 1980, Wegmann et al. 1998).
5. Host Relationships
It is often stated that a good parasite does not kill its host. That said, variation in the degree of pathogenicity exhibited by various parasitic plants is great, from those that exert little impact on their hosts (e.g. Epifagus on Fagus) to those that dramatically affect the host physiology and fecundity (e.g. Striga and Orobanche on various crop plants). Pathogenicity depends upon many factors, such as the biomass ratio of parasite to host, the number of parasites attached to an individual host plant, the length of time required for the parasite to complete its life cycle, and possibly the degree of coevolutionary "tuning" that has occurred over time between the two species. Complicating factors include examples such as Striga on cereals. Here the size of the parasite does not appear to be concordant with the degree that the host physiology is perturbed (Press et al. 1996) and is likely related to changes in host growth regulating (Drennan and El-Hiweris 1979). Reduction in host biomass is not explained simply by source-sink relationships, but by phytotoxic effects that change the partitioning of host photosynthate from shoot to root and an overall reduction in photosynthetic rate (Ransom et al. 1996). Despite variations in pathogenicity and life cycle dynamics, all parasitic plant species have evolved under the constraint that they do not kill their hosts prior to successful reproduction.
Before discussing host specificity, it is important to highlight two concepts: host range and host preference. The host preference of a parasite refers to those hosts that are parasitized in nature. When these parasites are presented with additional hosts under artificial conditions, parasitism may occur, thus indicating a broader host range. An example of this is Cuscuta epilinum which occurs naturally only on Linum, but will colonize Impatiens when given the opportunity (Kuijt 1969). In terms of host specificity, the full range from generalists to specialists can be found, sometimes among species of the same genus. For example, among the 45 species of Arceuthobium (dwarf mistletoes, Viscaceae), some parasitize only a single host species, such as A. apachecum on Pinus strobiformis, whereas others, such as A. globosum ssp. grandicaule, infects at least 12 host species (Hawksworth and Wiens 1996). A similar broad host range can be cited for various species of Cuscuta. Some Loranthaceae have especially wide host ranges, as exemplified by Dendrophthoe falcata which has been recorded from over 350 species and additional reports continue to increase this number (Joshi 1985, Narayanasamy 1981). Note that, however, recent work has shown that this widespread and polymorphic taxon is actually composed of several morphologically and geographically discrete species (Barlow 1995). The pantropical Cassytha filiformis (Lauraceae) appears to be totally indiscriminate in host choice, often covering and parasitizing dozens of host species simultaneously (Werth, 1979). Moreover, the parasite as often forms attachments to itself (autoparasitism) as it does to its host.
Parasite groups in which host specialization is the norm include Misodendraceae (Misodendrum occurs exclusively on Nothofagus), some Scrophulariaceae (Orobanchaceae, i.e. Conopholis on Quercus, Epifagus on Fagus, etc.) and Rafflesiales. For the latter order, all three genera of Rafflesiaceae s. str. (Rafflesia, Rhizanthes and Sapria) are known only from Tetrastigma (Vitaceae). Similar host specializations have evolved in the other segregate families of this order, e.g. Apodanthes on Flacourtiaceae, Pilostyles (including Berlinianche) on legumes, and Mitrastema on Fagaceae. One might predict that, like Rafflesiales, Balanophoraceae and Hydnoraceae would be similarly specialized along host lines, but such is not the case. One factor that may promote selection of host specificity is the occurrence of a parasite within a more homogeneous community (e.g. temperate vs. tropical forests), thus increasing the density of potential hosts (Kuijt 1969). Such an explanation does not apply to Rafflesiales, the majority of whose members occur in tropical forests with extremely high host diversity. Canalization along host lines, i.e. the "specialist strategy," likely has advantages when hosts are common, but from an evolutionary perspective, generalists likely persist longer in geologic time. This may explain why the majority of parasitic flowering plants are not host specialists.
6. Coevolutionary Relationships
One of the most remarkable coevolutionary relationships that exists is the presence of one parasitic angiosperm upon another. Two forms of association can be distinguished, facultative and obligate. As suggested by Wiens and Calvin (1987) the term hyperparasite should be used to describe a facultative association between different parasite species. Probably the most frequent and generalized examples of hyperparasitism involve Cuscuta and Cassytha. These genera parasitize a variety of plants which, by chance, may include woody root hemiparasites such as Ximenia (Olacaceae), Santalum (Santalaceae), etc. Cuscuta and Cassytha have also been reported as hyperparasites of mistletoes such as Phoradendron and Struthanthus (Kuijt 1964).
In contrast, the obligate situation called epiparasitism is known from mistletoes of both Loranthaceae, Viscaceae and Santalaceae in both the Paleotropics and Neotropics. The sole epiparasitic genus in the latter family is Phacellaria of southeast Asia which is an obligate parasite of Loranthaceae (Danser 1939). All possible host-parasite combinations have been reported (e.g. loranth on loranth, viscoid on viscoid, viscoid on loranth, and loranth on viscoid), although certain combinations are more common in particular regions. For example, Viscaceae have rarely been reported on Loranthaceae in the New World, a rare exception being Phoradendron iltisii on Cladocolea pringlei (Kuijt 1990). Approximately ten species of Phoradendron have been reported to be epiparasites, and indeed the entire 'Amplectens' group may be so (Kuijt 1987). At least ten species of epiparasitic Viscum have been documented from Australia and Asia (examples include V. articulatum and V. loranthi) and Africa (e.g. V. loranthicola) with Loranthaceae being the most frequent hosts. In Africa, Loranthaceae such as Agelanthus pungu are frequently found parasitizing other members of both Loranthaceae and Viscaceae (Polhill and Wiens 1998). New World loranth-loranth combinations can be found in Ixocactus, Notanthera, Phthirusa, and Tristerix, however, only one report of the inverse exists, i.e. Oryctanthus occidentalis on Phoradendron crassifolium (Kuijt 1964). Even more remarkable are tripartite epi- or hyperparasitic associations, such as Scurrula ferrugineus on Viscum articulatum on Elytranthe barnesii which was itself parasitic on Durio (Sands, 1924). Similarly, in South Africa, the following association has been reported: Viscum verrucosum on Tapinanthus quequensis (= T. leendertziae) on Agelanthus natalitius (= T. natalitius) upon Combretum apiculatum (Visser 1982). Field studies have shown that mistletoes maintain a water potential difference ca. 1000 kPa less than their nonparasitic hosts when both species are transpiring maximally. When an epiparasitic mistletoe is measured, its water potential is 1000 kPa less than its host mistletoe (Visser 1982). Water potential measurements have never been made on the component species in involved a three-way association, but the question can be asked "how great can water potential differences become?"
In addition to their relationships with their hosts, parasitic plants often develop complex associations with other organisms they encounter throughout their life cycle. Pollination and fruit/seed dispersal have been extensively reviewed elsewhere, hence only an overview will be presented here. A recent review of floral biology and reproductive ecology of parasitic plants can be found in (Molau 1995). Pollination involves the full spectrum of syndromes including: 1) beetles, e.g. Hydnoraceae (Musselman 1991) and Lophophytum (Balanophoraceae) (Borshenius and Olesen, 1990); 2) bees, e.g. Pedicularis (Scrophulariaceae) (Macior 1986), Krameria (Simpson, 1989), and Balanophora (Govindapa and Shivamurthy 1975) and Mystropetalon (Visser 1981); 3) flies, e.g. Rafflesia (Beaman et al. 1988), Rhizanthes (Bänziger 1996), Sarcophyte (Visser 1981); 4) birds, e.g many Loranthaceae (Reid 1990), Arjona, Quinchamalium (Santalaceae), Castilleja (Scrophulariaceae), and Mitrastema (Beehler 1994); and 5) bats, e.g. Dactylanthus (Ecroyd 1995).
As with pollination, seed dispersal syndromes are various. Among mistletoes, birds are important dispersal agents in most species of Loranthaceae and Viscaceae. Indeed, for the former family, the mistletoe birds (Dicaeidae) have highly specialized digestive tracts and behaviors that aid in dispersing loranth seeds (Docters van Leeuwen 1954, Reid 1990). Mammals are also involved in dispersing fruits and seeds of some parasitic plants, e.g. possums for Prosopanche (Cocucci and Cocucci 1996) and treeshrews (Tupaia) or plantain squirrels (Callosciurus) for Rafflesia (Emmons et al. 1991).
A significant yet poorly known aspect of parasitic plant biology is their association with microorganisms (fungi and bacteria). Atsatt (1973) suggested that haustoria were first produced in response to microbial parasitism and subsequently were modified for water and nutrient uptake. As mentioned in the Introduction, the gymnosperm Parasitaxus and its host root are both infected by a fungus that acts as a bridge between host and "parasite." This type of symbiotic association is almost certainly not homologous with (or ancestral to) haustorial parasitism in angiosperms, but it may represent an independent evolutionary experiment that approaches parasitism. Field observations have shown that seedlings of the holoparasite Conopholis americana (Scrophulariaceae) are only found attached to mycorrhizal roots of their oak host (Baird and Riopel 1980). Because essentially all oak roots found in nature are mycorrhizal, it is difficult to determine whether this association is obligate. Experimental studies using exudates from intact oak mycorrhizal roots yielded a 3% germination rate, comparable to rates seen from soil samples (Baird and Riopel 1986). Hemiparasitic Scrophulariaceae (e.g. Melampyrum) may also show a preference for mycorrhizal roots (Heinricher 1917). It has also been observed that Cuscuta preferentially parasitizes host plants that have mycorrhizal associations (Sanders et al. 1993). A number of fungi are associated with mistletoes, usually as facultative hyperparasites but obligate associations are also known such as Wallrothiella arceuthobii on Arceuthobium (see review in (Gill 1961).
7. Parasitic Plants and Human Activities
As discussed under "Host Relationships" above, it is useful to distinguish between a plant that is a parasite and one that is also a pathogen (i.e., causing disease). Such a distinction is a difficult one to make, for disease implies a condition where the normal host functions disrupted (Holliday 1989). Exactly how much functional alteration is required before a parasite can be called a pathogen is open to question and may require measurements of many parameters to determine the degree of deviation from the "normal" condition. In point of fact, parasitic plants occupy all possible positions along the symbiotic pathway from parasitism to commensalism. Pathogenicity also carries an antropocentric connotation in that one is more likely to call a parasitic plant a pathogen when it negatively impacts a host that has some economic importance.
How many genera of parasitic plants are pathogens of plants utilized by humans? As shown in Table 2, approximately 30 genera of parasitic angiosperms have been reported to negatively impact a host plant that is cultivated or harvested by humans. Given that there exists 274 genera, only about 11% of all genera have members that could be considered pathogens. This number is actually inflated, for indeed most of the damage inflicted upon economically valuable hosts is caused by just four genera: Cuscuta, Arceuthobium, Orobanche, and Striga. Arceuthobium represent an unusual case where a native pathogen inflicts a significant impact upon a natural forest community that is harvested for timber (Hawksworth and Wiens 1996). Although the other three genera do exist on native hosts, populations can explosively increase on monocultures of herbaceous crop plants. Despite the fact that only a tiny fraction of the total number of parasitic plants are pathogens on host plants used by humans, it must also be stated that the economic impact of the above four genera is enormous. For Arceuthobium, it is estimated that about 11.3 million cubic meters of wood are lost annually in the western U.S. and Canada valued at several billion dollars (Hawksworth and Wiens 1996). The witchweeds (particularly Striga asiatica, S. aspera, S. gesnerioides, and S. hermonthica) are the most significant parasitic weeds in the world, particularly in semi-arid regions of Africa and Asia (Riches and Parker 1995). Both the extent of infestation by Striga and the annual dollar amount lost are difficult to determine. One estimate indicates that of 67% of the 73 million ha placed in cereal production in sub-Saharan Africa is infested with Striga (Lagoke et al. 1991). In Northern Ghana alone, the economic loss from Striga on maize, millet and sorghum amounted to 25 million dollars in 1988 (Sauerborn 1991). As pointed out in this report, the focus on monetary terms presents an incomplete picture because the people operating these small farms rely directly on such grain crops for survival. The genus Orobanche includes four damaging pathogens: O. crenata, O. cernua, O. ramosa, and O. aegyptiaca. Several hundred thousand hectares are infested with Orobanche from Europe to the Middle East, Russia and China as well as Cuba and California (Riches and Parker 1995). Broomrape species are particular problems on crop plants in Apiaceae, Asteraceae, Brassicaceae, Cucurbitaceae, Fabaceae, and Solanaceae, hence they impact directly food destined for human consumption.
Conventional methods for controlling Striga and Orobanche, such as the use of herbicides, have not generally proven successful. For small-scale subsistence farmers in Africa, herbicides are not a financially practical solution to Striga control, hence they often resort to hand weeding, crop rotations, or fallow rotations. Alternatives involve varying agronomic methods (integrated control), the development of resistant crops (Kuiper et al. 1998), and biological control agents such as Fusarium oxysporium (Ciotola et al. 1995). More recently, it has been suggested that genetic engineering can be used to debilitate Striga by incorporating deleterious genes (Gressel 1999, Joel et al. 1995). Similar approaches are also being explored for Orobanche (Cubero et al. 1999, Lu et al. 1999, Thomas et al. 1999) and it has even been suggested (Rubiales 1999) that eating broomrape might be considered as part of the integrated control package! Ultimately, development of effective and low-cost control measures for Striga and Orobanche remains the "holy grail" for plant pathologists, agronomists, and biotechnologists.
Atsatt, P. R. 1973. Parasitic flowering plants: how did they evolve? American Naturalist 107: 502-510.
Baird, W. W., and J. L. Riopel. 1980. Studies on the life history of Conopholis americana (Orobanchaceae). Bot. Soc. Am. Misc. Ser. Publ. 159: 9.
Baird, W. W., and J. L. Riopel. 1986. Life history studies of Conopholis americana (Orobanchaceae). Amer. Midl. Nat. 116: 140-151.
Bänziger, H. 1996. Pollination of a flowering oddity: Rhizanthes zippelli (Blume) Spach (Rafflesiaceae). Nat. Hist. Bull. Siam Soc. 44: 113-142.
Barlow, B. 1995. New and noteworthy Malesian species of Loranthaceae. Blumea 40: 15-31.
Beaman, R. S., P. J. Decker, and J. H. Beaman. 1988. Pollination of Rafflesia (Rafflesiaceae). Amer. J. Bot. 75: 1148-1162.
Beehler, B. M. 1994. Canopy-dwelling honeyeater aggressively defends terrestrial nectar resource. Biotropica 26: 459-461.
Bjorkmann, E. 1960. Monotropa hypopitys L, an epiparasite on tree roots. Physiol. Plant. 13: 308-327.
Ciotola, M., A. K. Watson, and S. G. Hallett. 1995. Discovery of an isolate of Fusarium oxysporum with potential to control Striga hermonthica in Africa. Weed Res. 35: 303-309.
Cocucci, A. E., and A. A. Cocucci. 1996. Prosopanche (Hydnoraceae): somatic and reproductive structures, biology, systematics, phylogeny and potencialities as a parasitic weed. Pages 179-193 in M. T. Moreno, J. I. Cubero, D. Berner, D. Joel, L. J. Musselman, and C. Parker, eds. Advances in Parasitic Plant Research. Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, Spain.
Cubero, J. I., M. T. Moreno, D. Rubiales, and J. Sillero, eds. 1999. Resistance to Orobanche: The state of the art. Dirección General de Investigación y Formación Agraria, Sevilla, Spain.
Danser, B. H. 1939. A revision of the genus Phacellaria (Santalaceae). Blumea 3: 212-235.
Docters van Leeuwen, W. M. 1954. On the biology of some Javanese Loranthaceae and the role birds play in their life-history. Beufortia, Misc. Publ. 4: 105-207.
Drennan, D. S. H., and S. O. El-Hiweris. 1979. Changes in growth regulating substances in Sorghum vulgare infected by Striga hermonthica. Pages 144-155 in L. J. Musselman, A. D. Worsham, and R. E. Eplee, eds. Second International Symposium on Parasitic Weeds. North Carolina State University, Raleigh, NC, USA.
Ecroyd, C. E. 1995. Dactylanthus and bats: the link between two unique endangered New Zealand species and the role of the community in their survival. Pages 78-87 in D. A. Saunders, J. E. Craig, and E. M. Mattiske, eds. Nature Conservation 4: The Role of Networks. Surrey Beatty and Sons.
Emmons, L. H., J. Nais, and A. Briun. 1991. The fruit and consumers of Rafflesia keithii (Rafflesiaceae). Biotropica 23: 197-199.
Furman, T. E., and J. M. Trappe. 1971. Phylogeny and ecology of mycotrophic achlorophyllous angiosperms. Quart. Rev. Biol. 46: 219-225.
Gill, L. S. and. F. G. Hawksworth. 1961. The mistletoes, a literature review. U.S.D.A. Techn. Bull. No.1, 242 pages.
Govindapa, D. A., and G. R. Shivamurthy. 1975. The pollination mechanism in Balanophora abbreviata Blume. Ann. Bot. 39: 977-978.
Gressel, J. 1999. Tandem constructs:preventing the rise of superweeds. Trends in Biotechnology 17: 361-366.
Grieve, B. J. 1975. Botany in western Australia: a survey of progress: 1900-1971. J. Proc. R. Soc. West. Aust. 58: 33-53.
Hawksworth, F. G., and D. Wiens. 1996. Dwarf Mistletoes: Biology, Pathology and Systematics. USDA Forest Service, Washington D.C.
Heinricher, E. 1917. Zur Physiologie der schmarotzenden Rhinantheen, besonders der Halbparasiten. Naturwis. 5: 113-119.
Hocking, P. J. & B. A. Fineran. 1983. Aspects of the nutrition of root parasitic Loranthaceae. Pp. 230-257 in D. M. Calder & P. Bernhardt, eds., The Biology of Mistletoes. Academic Press, New York.
Holliday, P. 1989. A dictionary of plant pathology. Cambridge University Press, Cambridge.
Hull, R. J., and O. A. Leonard. 1964. Physiological aspects of parasitism in mistletoes (Arceuthobium and Phoradendron). 2. The photosynthetic capacity of mistletoes. Pl. Phys. 39: 1008-1017.
Joel, D. M., Y. Kleifeld, D. Losner-Goshen, G. Herzlinger, and J. Gressel. 1995. Transgenic crops against parasites. Nature 374: 220-221.
Joshi, G. C. P. C. P., and B. P. Kothyari. 1985. New host of Dendrophthoe falcata (Linn. f.) Etting. Ind. J. For. 8: 235.
Köpke, E., L. J. Musselman, and D. J. De Laubenfels. 1981. Studies on the anatomy of Parasitaxus ustus and its root connections. Phytomorph. 31: 85-92.
Kuijt, J. 1963. On the ecology and parasitism of the Costa Rican tree mistletoe, Gaiadendron punctatum (Ruiz and Pavón) G. Don. Can. J. Bot. 41: 927-938.
Kuijt, J. 1964. Critical observations on the parasitism of New World mistletoes. Can. J. Bot. 42: 1243-1278 .
Kuijt, J. 1969. The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA.
Kuijt, J. 1987. Novelties in mesoamerican mistletoes (Loranthaceae and Viscaceae). Ann. Missouri Bot. Gard. 74: 511-532.
Kuijt, J. 1990. New species and combinations in neotropical mistletoes (Loranthaceae and Viscaceae). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam. 93: 113-162.
Kuijt, J., J. H. Visser, and H.-C. Weber. 1978. Morphological observations on leaf haustoria and related organs of the South African genus Hyobanche (Scrophulariaceae). Can. J. Bot. 56: 2981-2986.
Kuiper, E. 1997. Comparative studies on the parasitism of Striga aspera and Striga hermonthica on tropical grasses. Pages 144. Vrije Universiteit Amsterdam, Amsterdam.
Kuiper, E., A. Groot, E. C. M. Noordopver, A. H. Pieterse, and J. A. C. Verkleij. 1998. Tropical grasses vary in their resistance to Striga aspera, Striga hermonthica, and their hybrids. Can. J. Bot. 76: 2131-2144.
Lagoke, S. T. O., V. Parkinson, and R. M. Agunbiade. 1991. Parasitic weeds and control methods in Africa. Pages 3-14 in S. K. Kim, ed. Combating Striga in Africa. IITA (International Institute of Tropical Agriculture), Ibadan, Nigeria.
Lavorel, S., M. S. Smith, and N. Reid. 1999. Spread of mistletoes (Amyema preissii) in fragmented Australian woodlands: a simulation study. Landscape Ecology 14: 147-160.
Lu, Y., G. Gagne, B. Grezes-Besset, and P. Blanchard. 1999. Integration of a molecular linkage group containing the broomrape resistance gene Or5 into an RFLP map in sunflower. Genome 42: 453- 456.
Machado, M. A., and K. Zetsche. 1990. A structural, functional and molecular analysis of plastids of the holoparsites Cuscuta reflexa and Cuscuta europaea. Planta 181: 91-96.
Macior, L. W. 1986. Floral resource sharing by bumblebees and hummingbirds in Pedicularis (Scrophulariaceae) pollination. Bull. Torrey Bot. Club 113: 101-109.
Main, A. 1947. Artificial propagation of Nuytsia floribunda. W. Austr. Natural. 1: 25-31.
Mann, W. F. Jr., and L. J. Musselman. 1981. Autotrophic growth of southern root parasites. American Midland Naturalist 106: 203-205.
McKinney, L. E., and T. E. Hemmerly. 1977. Host specificity of mistletoe in middle Tennessee III. Davidson county. J. Tennessee Acad. Sci. 52: 40.
Menzies, B. P. a. H. S. M. 1959. Root parasitism in Atkinsonia ligustrina (A. Cunn. ex F. Muell.) F. Muell. Proc. Linn. Soc. N. S. W. 84: 118-127.
Mohamed, K. I., L. J. Musselman, E. I. Aigbokhan, and D. K. Berner. 1996. Evolution and taxonomy of agriculturally important Striga species. Pages 53-73 in M. T. Moreno, J. I. Cubero, D. Berner, D. Joel, L. J. Musselman, and C. Parker, eds. Advances in Parasitic Plant Research. Junta de Andalucia, Dirección General de Investigación Agraria,, Cordoba, Spain.
Molau, U. 1995. Reproductive ecology and biology. Pages 141-176 in M. C. Press and J. D. Graves, eds. Parasitic Plants. Chapman and Hall, London.
Musselman, L. J. 1980. The biology of Striga, Orobanche, and other root-parasitic weeds. Ann. Rev. Phytopath. 18: 463-489.
Musselman, L. J. 1991. The genus Hydnora (Hydnoraceae). Pages 247-250 in J. K. Ransom, L. J. Musselman, A. D. Worsham, and C. Parker, eds. Proceedings of the 5th International Symposium of Parasitic Weeds. CIMMYT, Nairobi, Kenya.
Musselman, L. J., and M. C. Press. 1995. Introduction to parasitic plants. Pages 1-13 in M. C. Press and J. D. Graves, eds. Parasitic Plants. Chapman and Hall, London.
Narayanasamy, C., and R. Sampathkumar. 1981. Host parasite relationships of Dendrophthoe falcata (Linn. f.) Bettingh. (Loranthus longiflorus Desr.). Journal of the Bombay Natural History Society 78: 192-193.
Pate, J. S., N. J. Davidson, J. Kuo, and J. A. Milburn. 1990a. Water relations of the root hemiparasite Olax phyllanthi (Labill) R. Br. (Olacaceae) and its multiple hosts. Oecologia 84: 186-193..
Pate, J. S., J. Kuo, and N. J. Davidson. 1990b. Morphology and anatomy of the haustorium of the root hemiparasite Olax phyllanthi (Olacaceae), with special reference to the haustorial interface. Ann. Bot. 65: 425-436.
Polhill, R., and D. Wiens. 1998. Mistletoes of Africa. The Royal Botanic Gardens Kew, Richmond-Surrey, UK.
Press, M. C., A. L. Gurney, D. L. Frost, and J. D. Scholes. 1996. How does the parasitic angiosperm Striga hermonthica influence host growth and carbon relations? Pages 304-310 in M. T. Moreno, J. I. Cubero, D. Berner, D. Joel, L. J. Musselman, and C. Parker, eds. Advances in Parasitic Plant Research. Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, Spain.
Ransom, J. K., G. D. Odhiambo, R. E. Eplee, and A. O. Diallo. 1996. Estimates from field studies of the phytotoxic effects of Striga spp. on maize. Pages 327-333 in M. T. Moreno, J. I. Cubero, D. Berner, D. Joel, L. J. Musselman, and C. Parker, eds. Advances in Parasitic Plant Research. Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, Spain.
Reid, N. 1990. Mutualistic interdependence between mistletoes (Amyema quandang), spiny-cheeked honeyeaters and mistletoebirds in an arid woodland. . Austr. J. Ecology 15: 175-190.
Riches, C. R., and C. Parker. 1995. Parasitic plants as weeds. Pages 226-255 in M. C. Press and J. D. Graves, eds. Parasitic Plants. London, Chapman and Hall.
Rubiales, D. 1999. Eating broomrape? Pages 195-199 in J. I. Cubero, M. T. Moreno, D. Rubiales, and J. Sillero, eds. Resistance to Orobanche: The state of the art. Junta de Andalucía, Consejería de Agricultura y Pesca, Sevilla, Spain.
Sanders, I. R., R. T. Koide, and D. L. Shumway. 1993. Mycorrhizal stimulation of plant parasitism. Can. J. Bot. 71: 1143-1146.
Sauerborn, J. 1991. The economic importance of the phytoparasites Orobanche and Striga. Pages 137-143 in J. K. Ransom, L. J. Musselman, A. D. Worsham, and C. Parker, eds. Proceedings of the 5th. International Symposium of Parasitic Weeds. The International Maize and Wheat Improvement Center, Nairobi, Kenya.
Skeate, S. T. 1987. Interactions between birds and fruits in a northern florida hammock community. Ecology 68: 297-309.
Thomas, H., J. Sauerborn, D. Müller-Stöver, and J. Kroschel. 1999. Fungi of Orobanche aegyptiaca in Nepal with potential as biological control agents. Biocontrol Science and Technology 9: 379-381.
Visser, J. 1981. South African Parasitic Flowering Plants. Juta & Co., Lmt., Capetown.
Visser, J. H. 1982. Epiparasitism - how many mistletoes on one another? The Golden Bough (Royal Botanic Gardens, Kew) 1: 4.
Visser, J. H., H.-C. Weber, and J. Kuijt. 1978. Hyobanche sanguinea L. (Scrophulariaceae), ein Blattparasit. Naturwis. 65: 209.
Weber, H. C. 1981. Untersuchungen an parasitishen Scrophulariaceen (Rhinanthoideen) in Kulture. I. Keimung und Entwicklungsweise. Flora 171: 23-38.
Wegmann, K., L. J. Musselman, and D. M. Joel, eds. 1998. Current problems of Orobanche researches. Institute for Wheat and Sunflower "Dobroudja", Albena, Bulgaria.
Wiens, D., and C. L. Calvin. 1987. Epiparasitism in mistletoes. The Golden Bough (Royal Botanic Gardens Kew) 9: 3-5.
Woltz, P. R., A. Stockey, M. Gondran, and J. F. Cherrier. 1994. Interspecific parasitism in the gymnosperms: unpublished data on two endemic New Caledonian Podocarpaceae using scanning electron microscopy. Acta Bot. Gallica 141: 731-746.
| Family/Order |
Number Genera |
Number Species |
Parasitism Type | Example Genera | Distribution |
| Balanophoraceae | 17 | 43 | root, holo. | Balanophora, Corynaea, Scybalium, Thonningia | Pantropical |
| Convolvulaceae | 1 | 160 | stem, hemi. & holo. | Cuscuta | Worldwide |
| Cynomoriaceae | 1 | 1-2 | root, holo. | Cynomorium | S. Europe, N. Africa, C. Asia |
| Hydnoraceae | 2 | ca. 15 | root, holo. | Hydnora, Prosopanche | S. Amer., Africa, Madagascar |
| Krameriaceae | 1 | 17 | root, hemi. | Krameria | N. & S. America |
| Lauraceae | 1 | 20 | stem, hemi. | Cassytha | Pantropical |
| Lennoaceae | 2 | 5 | root, holo. | Lennoa, Pholisma | N. & S. America |
| Santalales | |||||
| 74 | 910 | stem & root, hemi. | Amyema, Phthirusa, Psittacanthus, Tapinanthus | Pantropical | |
| 1 | 8 | stem, hemi. | Misodendrum | S. America | |
| 29 | 193 | root, hemi. | Schoepfia, Ximenia | Pantropical | |
| 10 | 32 | root, hemi. | Agonandra, Opilia | Pantropical | |
| 38 | 490 | stem & root, hemi. | Comandra, Santalum, Thesium | Worldwide | |
| 7 | ca. 350 | stem, hemi. | Arceuthobium, Phoradendron, Viscum | Worldwide | |
|
Scrophulariaceae s. lat. (incl. Orobanchaceae) |
85 | ca. 1600 | root, hemi. & holo. | Agalinis, Buchnera, Castilleja, Epifagus, Euphrasia, Pedicularis, Orobanche, Rhinanthus, Striga | Worldwide |
| Rafflesiales | |||||
| 3 | 19 | stem & root, holo. | Rafflesia, Rhizanthes, Sapria | Malaya | |
| 2 | 7-11 | stem & root, holo. | Bdallophyton, Cytinus | Africa, Madagascar, Mexico, C. America | |
| 2-3 | 23 | stem, holo. | Apodanthes, Pilostyles | Africa, N., C. S. America, Middle East, Australia | |
| 1 | 2 | root, holo. | Mitrastema | C. America, Malaya to Japan | |
| Totals | 278 | 3900 |
| Parasite | Host | Distribution |
| Balanophoraceae | ||
| Thonningia sanguinea | Hevea | Nigeria |
| Balanophora indica | Coffea | India |
| Convolvulaceae | ||
| Cuscuta spp. | Various crops | Worldwide |
| Hydnoraceae | ||
| Prosopanche bonacinae | Gossypium | Argentina |
| Lauraceae | ||
| Cassytha filiformis | Various ornamentals | Pantropical |
| Santalaceae | ||
| Acanthosyris pauloalvimii | Theobroma | Brazil |
| Exocarpos spp. | Eucalyptus | Australia |
| Osyris alba | Vitis | Yugoslavia |
| Pyrularia pubera | Abies fraseri | West Virginia, USA |
| Thesium spp. | Saccharum, Hordeum, etc. | Australia, USA, Spain, Libya, S. Africa |
| Viscaceae | ||
| Arceuthobium spp. | Pinaceae (New World) & Cupressaceae (Old World) | USA, Europe, Asia, Africa |
| Dendrophthora poeppigii | Hevea | Brazil |
| Phoradendron spp. | various trees | North, Central and South America |
| Viscum spp. | various trees | Europe, Africa, Australia, Asia |
| Loranthaceae | ||
| Amyema spp. | Eucalyptus | Australia |
| Tapinanthus bangwensis | various trees | Africa |
| Dendrophthoe falcata | various trees | India |
| Phthirusa brasiliensis | Hevea | Brazil |
| Psittacanthus calyculatus | Citrus | Mexico |
| Struthanthus spp. | Coffea, Citrus, etc. | Central & South America |
| Scrophulariaceae | ||
| Aeginetia indica | Saccharum | India |
| Alectra spp. | Arachis, Vigna, Helianthus | Africa |
| Bartsia odontites | Medicago | Wisconsin, USA |
| Christisonia wightii | Saccharum | Philippines |
| Orobanche spp. | Various crops | Worldwide |
| Rhamphicarpa fistulosa | Arachis, Oryza | Africa |
| Rhinanthus serotinus | forage crops | Europe |
| Seymeria cassioides | Pinus | Southern USA |
| Striga spp. | Various crops, esp. Poaceae | Africa, Asia, Australia, USA |
* Pathogenic defined as negatively impacting a host plant that is cultivated or harvested by humans. Data compiled from: Musselman (1980), Gill, and Hawksworth (1961), Hawksworth, and Wiens (1996), Riches, and Parker (1995).
SIUC / College of Science / Parasitic Plant Connection
URL: http://www.parasiticplants.siu.edu/Chapter2.html
Last updated: 01-Oct-05 / dln