Phylogenetic Relationships of Parasitic
Flowering Plants
The number of times parasitism has evolved in flowering plants
(angiosperms) has long been debated. The closest non-parasitic
relatives for a number of lineages have been known for some time: Cassytha
with Lauraceae, Lennoaceae with Boraginaceae (or Ehretiaceae),
Orobanchaceae with "Scrophulariaceae" in the traditional sense, and Cuscuta
with Convolvulaceae.
For others, particularly the holoparasites in the traditionally
recognized families Hydnoraceae, Balanophoraceae, and Rafflesiaceae,
placement among photosynthetic angiosperms has been difficult. For this
reason, traditional classifications were often conflicted among different
workers and even in different treatments by the same worker. Relationships
between parasitic and nonparasitic angiosperms have been greatly clarified
through DNA sequencing and molecular phylogenetic analyses, although not
without some "bumps in the road." One molecular evolutionary phenomenon
that has made phylogenetic work on the holoparasites less than
straightforward has been horizontal gene transfer, particularly of
mitochondrial genes. This has generated conflicts between gene trees
derived from the different subcellular genomes. A good example of this is
the paper by Barkman et al. (2007) who used molecular methods but could
not given an exact number for the origins of parasitism ("at least 11").
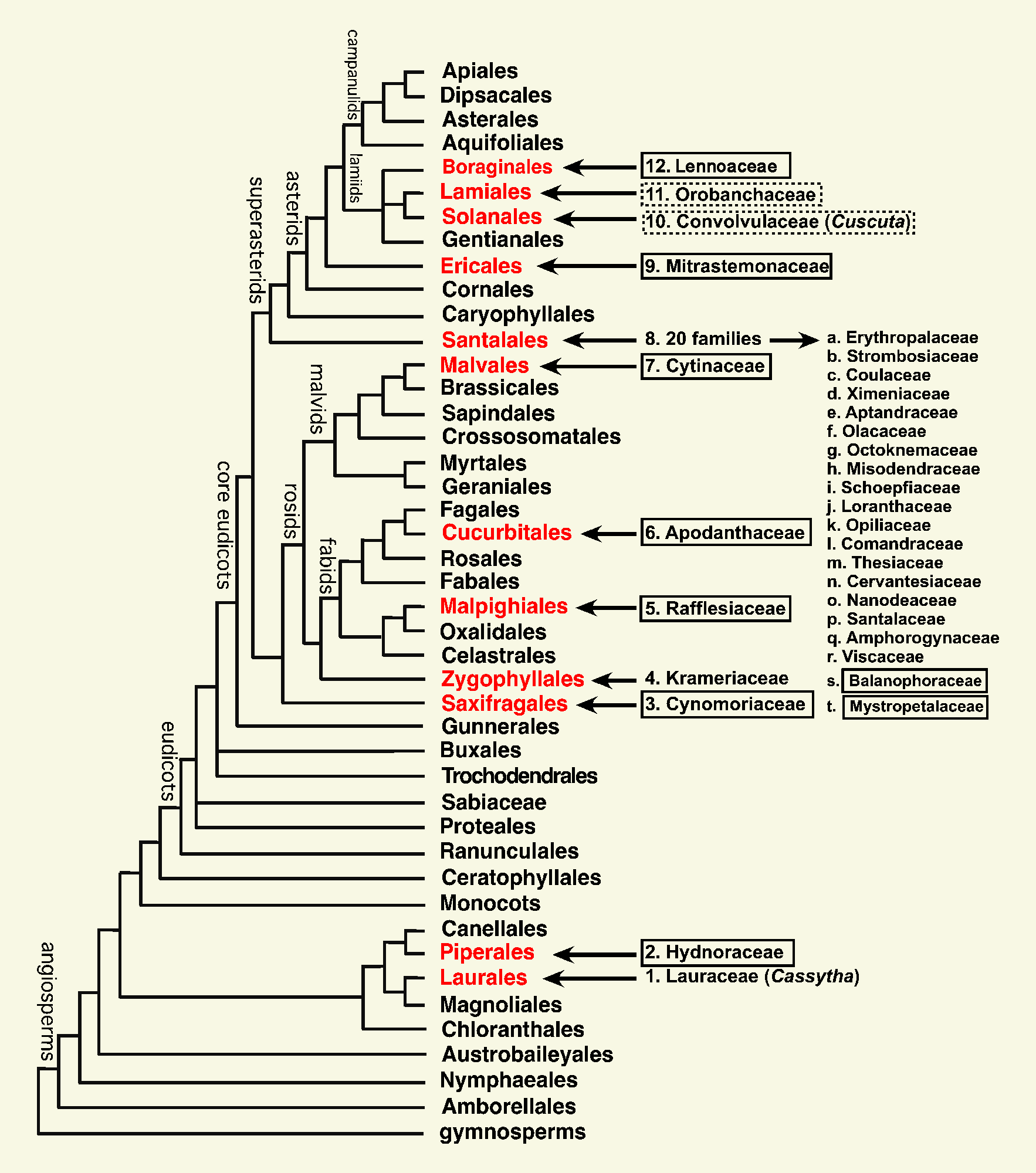
Currently, all clades of parasitic plants have been placed on the
global angiosperm phylogenetic tree. As described
below, parasitism has arisen independently in angiosperms 12
times.
In terms of trophic modes, 9 lineages (clades, families) are composed
entirely of holoparasites (family names enclosed in solid line
rectangles). Only two lineages (Cassytha
and Krameriaceae) contain just hemiparasites. In two families,
Convolvulaceae (Cuscuta) and
Orobanchaceae, both hemi- and holoparasites be found (family names
enclosed in dashed line rectangles). Only the order Santalales has
more than one family of parasitic plants.
Click on the family names to go to those pages
1. Laurales. Cassytha, the sole
parasitic member of the large family Lauraceae, is uniquivocally assigned
to this family based on morphological and molecular data (Rohwer &
Rudolph 2005). Its superficial resemblance to Cuscuta is
remarkable and an excellent example of convergent evolution.
2. Piperales. Molecular data were used to
place Hydnoraceae with Aristolochiaceae s. lat. (Nickrent
et
al. 2002), however, the exact topology of the component families of
this order (Aristolochiaceae, Hydnoraceae, Lactoridaceae, Piperaceae and
Saururaceae) was not determined. The analysis by Nickrent
(2005,
IBC abstract) suggested Hydnoraceae was most closely related to
Aristolochiaceae. This result was confirmed by more recent work (Naumann
et al. 2013). See bottom of the Hydnoraceae page HERE
for a discussion of why this family is being recognized here instead of
lumping into Aristolochiaceae (as was done by APG III).
3. Saxifragales. Although previous
classified with Balanophoraceae, the family Cynomoriaceae has been shown
to be a component of Saxifragales (Nickrent
et
al. 2005). For a more up-to-date and complete discussion
of this finding, see the Supplement to Su
et al. (2015) HERE.
This placement within Saxifralaes was confirmed by Bellot et al. (2016).
4. Zygophyllales. The APG2
classification considered Krameriaceae as an acceptable monophyletic
alternative to Zygophyllaceae. In APG3 the two families were
considered separate. This order is sister to the Fabid (previously
Rosid I) clade.
5. Rafflesiaceae. This family, considered
here in the strict sense (including Rafflesia, Rhizanthes,
and Sapria, i.e., the "large-flowered clade") was placed with
Malpighiales by Barkman et al. (2004) using mitochondrial matR gene
sequences. This position was confirmed by Nickrent
et al. (2004) using both nuclear SSU rDNA as well as mitochondrial
sequence data. Placement within the order shows that Rafflesiaceae is near
Euphorbiaceae (Davis
et al. 2007).
6. Apodanthaceae. Traditionally placed in
Rafflesiaceae, the "small-flowered clade" is composed of Apodanthes,
Berlinianche, and Pilostyles. Mitochondrial matR and nuclear
SSU rDNA data indicated either a relationship with Malvales or
Cucurbitales (Nickrent
et
al.
2004). Additional sequencing and analyses indicated that this family
is part of Cucurbitales. That result was confirmed by Filipowicz &
Renner (2010).
7. Cytinaceae. Traditionally placed
in Rafflesiaceae, the"inflorescence clade" is composed of Cytinus
and Bdallophyton. Mitochondrial matR and nuclear SSU rDNA both
strongly support a position for this family in Malvales (Nickrent
et
al. 2004).
8. Santalales. The analyses conducted by
Soltis et al. (2000) resolved the sandalwood order as monophyletic, but
this clade was part of a large polytomy among the core eudicots. The
number of taxa involved in that polytomy was quite high, involving
caryophyllids, rosids and asterids. A molecular analysis using complete
chloroplast genomes from over 80 angiosperms (Moore et al. 2010) indicated
that Santalales is sister to Caryophyllales and asterids (as shown in the
above tree).
The number of families recognized for Santalales on this web
site (20 total) follows Nickrent
et al. (2010) and Su
et al. (2015). Although the families segregated from Olacaceae s.
lat. and recognized by Malécot
& Nickrent (2008) were for the most part accepted by APG III
(2009), the segregate families of Santalaceae s. lat. were not, despite
the evidence presented in Der
& Nickrent (2008). With regard to Santalaceae,
Christenhusz et al. (2015) said "... in the future perhaps expand
this family to include the majority of Santalales, apart from
Balanophoraceae." From my perspective, this tendency towards extreme
lumping is excessive, unjustified, and provides no scientific advancement.
Moreover, this rash statement was made in the absence of information on
the phylogenetic status of Balanophoraceae s. lat. (see below).
The holoparasite family Balanophoraceae, previously placed
in its own order Balanophorales (Takhtajan 1997), has been shown from
molecular evidence (Nickrent
et
al. 2005) to be related to Santalales. A detailed molecular
phylogenetic analysis of the entire order was reported in Su
et al (2015) and in that study Balanophoraceae was not monophyletic.
Three genera, Dactylanthus,
Hachettea, and Mystropetalon, emerged
in a separate clade and are therefore classified in a separate family
Mystropetalaceae.
9. Mitrastemonaceae. Traditionally
placed in Rafflesiaceae, this monogeneric family (Mitrastemon) was
shown to be related to Ericales by Barkman et al. (2004) using
mitochondrial matR gene sequences. This result is confirmed using nuclear
SSU rDNA and mitochondrial sequence data (Nickrent
et
al. 2004).
11. Orobanchaceae. This family name
traditionally referred only to an assemblage of holoparasitic taxa that
were recognized to be related to the hemiparasites of Scrophulariaceae.
Modern circumscriptions of this group (see Young et al. 1999, Olmstead et
al. 2001) place all parasitic "scrophs" in a monophyletic family
Orobanchaceae along with the non-parasite Lindenbergia.
Morphological and molecular evidence clearly place this family in
Lamiales.
10. Convolvulaceae. The sole parasitic
genus of Convolvulaceae is Cuscuta that has sometimes been placed
in its own family, Cuscutaceae. Analysis of sequence data from four
chloroplast gene regions resulted in Cuscuta being nested within
Convolvulaceae (Stefanovic et al. 2002), thus the classification of APG
III (2009) is supported.
12. Lennoaceae. These
holoparasites have traditionally been placed in their own family,
but APG III lumped Lennoaceae with Boraginaceae, a family with
which they are clearly related as shown by both morphological and
molecular evidence. The genera Lennoa
and Pholisma were shown to be a
component of a monophyletic Ehretiaceae by Gottschling et al. (2014).
More recently, Luebert et al. (2016) provided a familial
classification for Boraginales and retained the family Lennoaceae, sister
to Ehretiaceae.
Literature Cited
APG III. 2009. An update of the Angiosperm Phylogeny Group
classification for the orders and families of flowering plants. Bot. J.
Linn. Soc. 161:105-121.
Barkman, T.J., Lim, S.-H., Mat Salleh, K., & Nais, J. 2004.
Mitochondrial DNA sequences reveal the photosynthetic relatives of Rafflesia,
the world's largest flower. Proc Natl Acad Sci U S A 101:787-792.
Barkman, T.J., McNeal, J.R., Lim, S.-H., Coat, G., Croom, H.B., Young,
N.D., & dePamphilis, C.W. 2007. Mitochondrial DNA suggests
at least 11 origins of parasitism in angiosperms and reveals genomic
chimerism in parasitic plants. B.M.C. Evol. Biol. 7:248.
Bellot S, Cusimano N, Luo S, Sun G, Zarre S, Groger A, Temsch E, Renner
SS. 2016. Assembled plastid and mitochondrial genomes, as well as nuclear
genes, place the parasite family Cynomoriaceae in the Saxifragales. Genome
Biology and Evolution 8:2214–2230.
Christenhusz, M.J.M., Vorontsova, M.S., Fay, M.F., & Chase, M.W. 2015.
Results from an online survey of family delimitation in angiosperms and
ferns: recommendations to the Angiosperm Phylogeny Group for thorny
problems in plant classification. Bot. J. Linn. Soc. 178:501-528.
Davis, C.C., Latvis, M., Nickrent, D.L., Wurdack, K.J., & Baum, D.A.
2007. Floral gigantism in Rafflesiaceae. Science 315:1812.
Der, J. P. and Nickrent, D. L. 2008. A molecular phylogeny of Santalaceae
(Santalales). Syst. Bot. 33: 107-116.
Filipowicz, N., & Renner, S.S. 2010. The worldwide holoparasitic
Apodanthaceae confidently placed in the Cucurbitales by nuclear and
mitochondrial gene trees. BMC Evol. Biol. 10:48.
Gottschling, M., Luebert, F., Hilger, H.H., & Miller, J.S. 2014.
Molecular delimitations in the Ehretiaceae (Boraginales). Mol. Phylog.
Evol. 72:1-6.
Luebert, F. and 20 others. 2016. Familial classification of the
Boraginales. Taxon 65(3): 502-522.
Malécot, V. and Nickrent, D. L. 2008. Molecular phylogenetic relationships
of Olacaceae and related Santalales. Syst. Bot. 33: 97-106.
Moore, M.J., Soltis, P.S., Bell, C.D., Burleigh, J.G., & Soltis, D.E.
2010. Phylogenetic analysis of 83 plastid genes further resolves the early
diversification of eudicots. Proc Natl Acad Sci U S A 107:4623-4628.
Naumann, J., Salomo, K., Der, J.P., Wafula, E.K., Bolin, J.F., Maass, E.,
Frenzke, L., Samain, M.-S., Neinhuis, C., dePamphilis, C.W., & Wanke,
S. 2013. Single-copy nuclear genes place haustorial Hydnoraceae within
Piperales and reveal a Cretaceous origin of multiple parasitic angiosperm
lineages. PLoS ONE 8:e79204.
Nickrent, D. L, A. Blarer, Y.-L. Qiu, R. Vidal-Russell, F. E. Anderson.
2004. Phylogenetic inference in Rafflesiales: the influence of rate
heterogeneity and horizontal gene transfer. BMC Evol. Biol. 4: 40.
Nickrent, D. L., A. Blarer, Y.-L. Qiu, D. E. Soltis, P. S. Soltis, and M.
Zanis. 2002. Molecular data place Hydnoraceae with Aristolochiaceae. Amer.
J. Bot. 89 (11): 1809-1817.
Nickrent, D.L., Der, J.P., & Anderson, F.E. 2005.
Discovery of the photosynthetic relatives of the "Maltese mushroom"
Cynomorium. BMC Evol. Biol. 5:
38.
Nickrent, D. L. V. Malécot, R. Vidal-Russell, and J. P. Der. 2010. A
revised classification of Santalales. Taxon 59: 538-558.
Olmstead, R.G., dePamphilis, C.W., Wolfe, A.D., Young, N.D., Elisens,
W.J., & Reeves, P.J. 2001. Disintegration of the Scrophulariaceae.
Amer. J. Bot. 88:348-361.
Rohwer, J.G., & Rudolph, B. 2005. Jumping genera: the phylogenetic
positions of Cassytha, Hypodaphnis,
and Neocinnamomum (Lauraceae)
based on different analyses of trnK intron sequences. Ann. Mo. Bot. Gard.
92:153-178.
Soltis, D.E., Soltis, P.S., Chase, M.W., Mort, M.E., Albach, D.C., Zanis,
M., Savolainen, V., Hahn, W.H., Hoot, S.B., Fay, M.F., Axtell, M.,
Swensen, S.M., Prance, L.M., Kress, W.J., Nixon, K.C., & Farris, J.S.
2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB
sequences. Bot. Jour. Linn. Soc. 133:381-461.
Stefanovi?, S., Krueger, L., & Olmstead, R.G. 2002. Monophyly of the
Convolvulaceae and circumscription of their major lineages based on DNA
sequences of multiple chloroplast loci. Amer. J. Bot. 89:1510-1522.
Su H.-J., J.-M. Hu, F. E. Anderson and D. L. Nickrent. 2015.
Phylogenetic relationships of Santalales with insights into the origins of
holoparasitic Balanophoraceae. Taxon 64(3): 491-506.
Takhtajan, A. 1997. Diversity and classification of flowering plants.
Columbia University Press, New York, NY.
Young, N.D., Steiner, K.E., & dePamphilis, C.W. 1999. The evolution of
parasitism in Scrophulariaceae/ Orobanchaceae: Plastid gene sequences
refute an evolutionary transition series. Ann. Mo. Bot. Gard. 86:876-893.

Last updated: